当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Graphene Oxide as Metal-Free Catalyst in Oxidative Dehydrogenative C–N Coupling Leading to α-Ketoamides: Importance of Dual Catalytic Activity
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acssuschemeng.7b02267 Biju Majumdar 1 , Daisy Sarma 1 , Tamalika Bhattacharya 1 , Tridib K. Sarma 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2017-09-05 00:00:00 , DOI: 10.1021/acssuschemeng.7b02267 Biju Majumdar 1 , Daisy Sarma 1 , Tamalika Bhattacharya 1 , Tridib K. Sarma 1
Affiliation
|
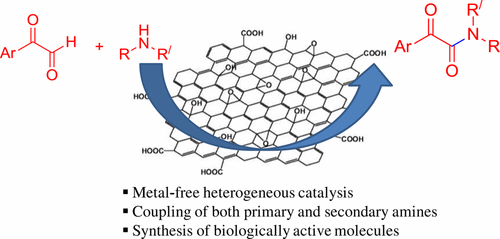
A heterogeneous, inexpensive, and environmentally friendly carbocatalyst, graphene oxide (GO) enables the formation of α-ketoamides from activated aldehydes and amines through a cross-dehydrogenative coupling pathway. The oxygenated functionalities (e.g., carboxyl, hydroxyl, ketonic, and epoxides) on the surface of graphene oxide impart acidic as well as oxidizing properties to the material. This dual catalytic property of graphene oxide is explored toward the generation of α-ketoamides where surface acidity of graphene oxide favors the initial formation of hemiaminal intermediate followed by oxidation leading to the desired final product. The hemiaminal intermediate could be isolated and confirmed by NMR and mass analysis. A few control experiments confirmed that both acidic and oxidizing catalytic activities of graphene oxide were instrumental in the coupling reaction. Use of benzoic acid and p-toluene sulfonic acid as catalysts resulted in the formation of only hemiaminal intermediate as the major product along with a trace amount of α-ketoamide. As these catalysts do not possess oxidative catalytic capability, the formation of ketoamide was not favorable. However, use of GO as the catalyst could generate the ketoamide product from the hemiaminal intermediate as initial acid-catalyzed hemiaminal formation was followed by oxidation to α-ketoamide. Graphene oxide annealed at different temperatures demonstrates the role of oxygenated functional groups in the catalytic reaction. Further investigation of catalytic activity with modified GO surfaces using various conditions, such as base, acid, or NaBH4, showed carboxylic acid groups on the surface to be the active site for the catalytic reaction. The method is also effective toward the synthesis of biologically important α-ketoamide.
中文翻译:

氧化石墨烯作为氧化性脱氢C-N偶联剂中的无金属催化剂,导致α-酮酰胺:双重催化活性的重要性
异质,廉价且环保的碳催化剂,氧化石墨烯(GO)可以通过交叉脱氢偶联途径由活化的醛和胺形成α-酮酰胺。氧化石墨烯表面上的氧化官能团(例如羧基,羟基,酮和环氧化物)赋予材料酸性和氧化性。氧化石墨烯的这种双重催化性能被研究用于生成α-酮酰胺,其中氧化石墨烯的表面酸性有利于半乳糖中间体的初始形成,然后氧化生成所需的最终产物。可以通过NMR和质量分析分离并鉴定出半胱氨酸中间体。一些对照实验证实,氧化石墨烯的酸性和氧化催化活性都对偶联反应有帮助。使用苯甲酸和对甲苯磺酸作为催化剂仅与主要的产物半乳糖醛中间体一起形成痕量的α-酮酰胺。由于这些催化剂不具有氧化催化能力,因此酮酰胺的形成是不利的。然而,使用GO作为催化剂可以从半胱氨酸中间体产生酮酰胺产物,因为最初的酸催化的半胱氨酸形成随后被氧化成α-酮酰胺。在不同温度下退火的氧化石墨烯证明了氧化官能团在催化反应中的作用。使用碱,酸或NaBH 4等各种条件进一步研究修饰的GO表面的催化活性,表明在表面上的羧酸基团是催化反应的活性部位。该方法对于合成生物学上重要的α-酮酰胺也是有效的。
更新日期:2017-09-05
中文翻译:

氧化石墨烯作为氧化性脱氢C-N偶联剂中的无金属催化剂,导致α-酮酰胺:双重催化活性的重要性
异质,廉价且环保的碳催化剂,氧化石墨烯(GO)可以通过交叉脱氢偶联途径由活化的醛和胺形成α-酮酰胺。氧化石墨烯表面上的氧化官能团(例如羧基,羟基,酮和环氧化物)赋予材料酸性和氧化性。氧化石墨烯的这种双重催化性能被研究用于生成α-酮酰胺,其中氧化石墨烯的表面酸性有利于半乳糖中间体的初始形成,然后氧化生成所需的最终产物。可以通过NMR和质量分析分离并鉴定出半胱氨酸中间体。一些对照实验证实,氧化石墨烯的酸性和氧化催化活性都对偶联反应有帮助。使用苯甲酸和对甲苯磺酸作为催化剂仅与主要的产物半乳糖醛中间体一起形成痕量的α-酮酰胺。由于这些催化剂不具有氧化催化能力,因此酮酰胺的形成是不利的。然而,使用GO作为催化剂可以从半胱氨酸中间体产生酮酰胺产物,因为最初的酸催化的半胱氨酸形成随后被氧化成α-酮酰胺。在不同温度下退火的氧化石墨烯证明了氧化官能团在催化反应中的作用。使用碱,酸或NaBH 4等各种条件进一步研究修饰的GO表面的催化活性,表明在表面上的羧酸基团是催化反应的活性部位。该方法对于合成生物学上重要的α-酮酰胺也是有效的。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号