当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiospecific Solvolytic Functionalization of Bromochlorides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-18 , DOI: 10.1021/jacs.7b07792 Alexander J Burckle 1 , Bálint Gál 1 , Frederick J Seidl 1 , Vasil H Vasilev 1 , Noah Z Burns 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-18 , DOI: 10.1021/jacs.7b07792 Alexander J Burckle 1 , Bálint Gál 1 , Frederick J Seidl 1 , Vasil H Vasilev 1 , Noah Z Burns 1
Affiliation
|
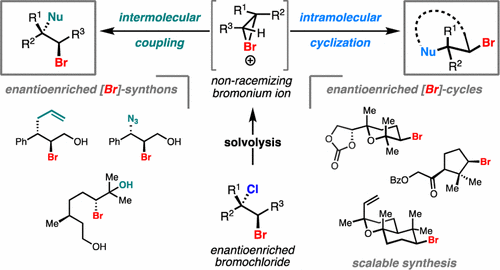
Herein, we report that under mild solvolytic conditions, enantioenriched bromochlorides can be ionized, stereospecifically cyclized to an array of complex bromocyclic scaffolds, or intermolecularly trapped by exogenous nucleophiles. Mechanistic investigations support an ionic mechanism wherein the bromochloride serves as an enantioenriched bromonium surrogate. Several natural product-relevant motifs are accessed in enantioenriched form for the first time with high levels of stereocontrol, and this technology is applied to the scalable synthesis of a polycyclic brominated natural product. Arrays of nucleophiles including olefins, alkynes, heterocycles, and epoxides are competent traps in the bromonium-induced cyclizations, leading to the formation of enantioenriched mono-, bi-, and tricyclic products. This strategy is further amenable to intermolecular coupling between cinnamyl bromochlorides and a diverse set of commercially available nucleophiles. Collectively, this work demonstrates that enantioenriched bromonium chlorides are configurationally stable under solvolytic conditions in the presence of a variety of functional groups.
中文翻译:

溴氯化物的对映特异性溶剂解官能化
在此,我们报告在温和的溶剂分解条件下,对映体富集的溴氯化物可以被电离,立体特异性环化为一系列复杂的溴环支架,或被外源亲核试剂分子间捕获。机理研究支持离子机理,其中溴氯化物充当对映体富集的溴替代品。首次以高水平立体控制的对映体富集形式获得了几种与天然产物相关的基序,并且该技术应用于多环溴化天然产物的可扩展合成。包括烯烃、炔烃、杂环和环氧化物在内的一系列亲核试剂是溴诱导环化反应中的有效陷阱,导致形成对映体富集的单环、双环和三环产物。该策略进一步适用于肉桂基溴氯化物和多种市售亲核试剂之间的分子间偶联。总的来说,这项工作证明了对映体富集的氯化溴在溶剂分解条件下和多种官能团存在下是构型稳定的。
更新日期:2017-09-18
中文翻译:

溴氯化物的对映特异性溶剂解官能化
在此,我们报告在温和的溶剂分解条件下,对映体富集的溴氯化物可以被电离,立体特异性环化为一系列复杂的溴环支架,或被外源亲核试剂分子间捕获。机理研究支持离子机理,其中溴氯化物充当对映体富集的溴替代品。首次以高水平立体控制的对映体富集形式获得了几种与天然产物相关的基序,并且该技术应用于多环溴化天然产物的可扩展合成。包括烯烃、炔烃、杂环和环氧化物在内的一系列亲核试剂是溴诱导环化反应中的有效陷阱,导致形成对映体富集的单环、双环和三环产物。该策略进一步适用于肉桂基溴氯化物和多种市售亲核试剂之间的分子间偶联。总的来说,这项工作证明了对映体富集的氯化溴在溶剂分解条件下和多种官能团存在下是构型稳定的。































 京公网安备 11010802027423号
京公网安备 11010802027423号