当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of Nitrile Hydratase Regioselectivity towards Dinitriles by Tailoring the Substrate Binding Pocket Residues
ChemCatChem ( IF 3.8 ) Pub Date : 2017-12-06 , DOI: 10.1002/cctc.201701170 Zhongyi Cheng 1 , Wenjing Cui 1 , Yuanyuan Xia 1 , Lukasz Peplowski 2 , Michihiko Kobayashi 3 , Zhemin Zhou 1
ChemCatChem ( IF 3.8 ) Pub Date : 2017-12-06 , DOI: 10.1002/cctc.201701170 Zhongyi Cheng 1 , Wenjing Cui 1 , Yuanyuan Xia 1 , Lukasz Peplowski 2 , Michihiko Kobayashi 3 , Zhemin Zhou 1
Affiliation
|
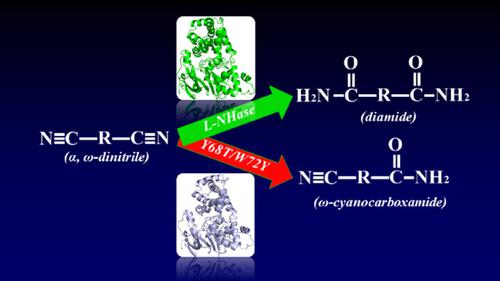
The regioselective hydration of dinitriles is one of the most attractive approaches to prepare ω‐cyanocarboxamides or diamides and such regioselectivity is often beyond the capability of chemical catalysts. The use of nitrile hydratase to biotransform dinitriles selectively would be highly desirable. Molecular docking of two aliphatic dinitriles and two aromatic dinitriles into the active site of a nitrile hydratase (NHase) from Rhodococcus rhodochrous J1 allowed the identification of proximal NHase substrate binding pocket residues. Four residues (βLeu48, βPhe51, βTyr68, and βTrp72) were selected for single‐ and double‐point mutations to modulate the NHase regioselectivity towards dinitriles. Several NHase mutants with an altered regioselectivity were obtained, and the best one was Y68T/W72Y. Docking experiments further indicated that the poor binding affinity of aliphatic and aromatic ω‐cyanocarboxamides to the NHase variants resulted in distinct regioselectivity between wild‐type and mutated NHases.
中文翻译:

通过定制底物结合口袋残基来调节腈水合酶对二腈的区域选择性
二腈的区域选择性水合是制备ω-氰基羧酰胺或二酰胺的最有吸引力的方法之一,这种区域选择性通常超出了化学催化剂的能力。非常需要使用腈水合酶来选择性地生物转化二腈。两个脂族二腈和两个芳族二腈分子对接至红球红球菌腈水合酶(NHase)的活性位点J1允许鉴定近端NHase底物结合口袋残基。选择了四个残基(βLeu48,βPhe51,βTyr68和βTrp72)进行单点和双点突变,以调节NHase对二腈的区域选择性。获得了几个区域选择性改变的NHase突变体,最好的是Y68T / W72Y。对接实验进一步表明,脂肪族和芳香族ω-氰基羧酰胺与NHase变异体的弱结合亲和力导致野生型和突变型NHases的区域选择性不同。
更新日期:2017-12-06
中文翻译:

通过定制底物结合口袋残基来调节腈水合酶对二腈的区域选择性
二腈的区域选择性水合是制备ω-氰基羧酰胺或二酰胺的最有吸引力的方法之一,这种区域选择性通常超出了化学催化剂的能力。非常需要使用腈水合酶来选择性地生物转化二腈。两个脂族二腈和两个芳族二腈分子对接至红球红球菌腈水合酶(NHase)的活性位点J1允许鉴定近端NHase底物结合口袋残基。选择了四个残基(βLeu48,βPhe51,βTyr68和βTrp72)进行单点和双点突变,以调节NHase对二腈的区域选择性。获得了几个区域选择性改变的NHase突变体,最好的是Y68T / W72Y。对接实验进一步表明,脂肪族和芳香族ω-氰基羧酰胺与NHase变异体的弱结合亲和力导致野生型和突变型NHases的区域选择性不同。













































 京公网安备 11010802027423号
京公网安备 11010802027423号