当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Co11Li[(OH)5O][(PO3OH)(PO4)5], a Lithium-Stabilized, Mixed-Valent Cobalt(II,III) Hydroxide Phosphate Framework
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01152 Jennifer Ludwig 1 , Stephan Geprägs 2 , Dennis Nordlund 3 , Marca M. Doeff 4 , Tom Nilges 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01152 Jennifer Ludwig 1 , Stephan Geprägs 2 , Dennis Nordlund 3 , Marca M. Doeff 4 , Tom Nilges 1
Affiliation
|
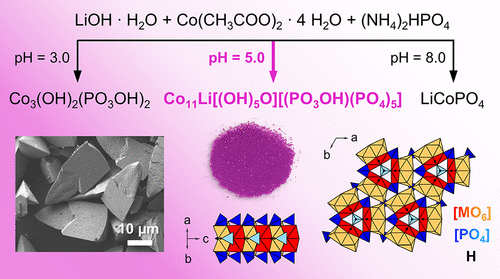
A new metastable phase, featuring a lithium-stabilized mixed-valence cobalt(II,III) hydroxide phosphate framework, Co11.0(1)Li1.0(2)[(OH)5O][(PO3OH)(PO4)5], corresponding to the simplified composition Co1.84(2)Li0.16(3)(OH)PO4, is prepared by hydrothermal synthesis. Because the pH-dependent formation of other phases such as Co3(OH)2(PO3OH)2 and olivine-type LiCoPO4 competes in the process, a pH value of 5.0 is crucial for obtaining a single-phase material. The crystals with dimensions of 15 μm × 30 μm exhibit a unique elongated triangular pyramid morphology with a lamellar fine structure. Powder X-ray diffraction experiments reveal that the phase is isostructural with the natural phosphate minerals holtedahlite and satterlyite, and crystallizes in the trigonal space group P31m (a = 11.2533(4) Å, c = 4.9940(2) Å, V = 547.70(3) Å3, Z = 1). The three-dimensional network structure is characterized by partially Li-substituted, octahedral [M2O8(OH)] (M = Co, Li) dimer units which form double chains that run along the [001] direction and are connected by [PO4] and [PO3(OH)] tetrahedra. Because no Li-free P31m-type Co2(OH)PO4 phase could be prepared, it can be assumed that the Li ions are crucial for the stabilization of the framework. Co L-edge X-ray absorption spectroscopy demonstrates that the cobalt ions adopt the oxidation states +2 and +3 and hence provides further evidence for the incorporation of Li in the charge-balanced framework. The presence of three independent hydroxyl groups is further confirmed by infrared spectroscopy. Magnetization measurements imply a paramagnetic to antiferromagnetic transition at around T = 25 K as well as a second transition at around 9–12 K with a ferromagnetic component below this temperature. The metastable character of the phase is demonstrated by thermogravimetric analysis and differential scanning calorimetry, which above 558 °C reveal a two-step decomposition to CoO, Co3(PO4)2, and olivine-type LiCoPO4 with release of water and oxygen.
中文翻译:

Co 11 Li [(OH)5 O] [(PO 3 OH)(PO 4)5 ],一种锂稳定的混合价氢氧化钴(II,III)磷酸盐骨架
一种新的亚稳态相,具有锂稳定的混合价钴(II,III)氢氧化钴磷酸盐骨架,Co 11.0(1) Li 1.0(2) [(OH)5 O] [(PO 3 OH)(PO 4)通过水热合成制备对应于简化组成Co 1.84(2) Li 0.16(3)(OH)PO 4的图5 ] 。因为其他相(例如Co 3(OH)2(PO 3 OH)2和橄榄石型LiCoPO 4)的pH依赖性形成竞争过程中,pH值5.0对于获得单相材料至关重要。尺寸为15μm×30μm的晶体表现出独特的细长三角锥形态,具有层状精细结构。粉末X射线衍射实验表明,该相与天然磷酸盐矿物holtedahlite和satterlyite是同构的,并且在三角空间群P 31 m(a = 11.2533(4)Å,c = 4.9940(2)Å,V = 547.70(3)3,Z = 1)。三维网络结构的特征在于部分被Li取代的八面体[M 2 O 8(OH)](M = Co,Li)二聚体单元,其形成沿[001]方向延伸的双链并通过[PO 4 ]和[PO 3(OH)]四面体连接。因为没有无锂的P 31 m型Co 2(OH)PO 4可以准备阶段,可以假设锂离子对于稳定框架至关重要。Co L边缘X射线吸收光谱表明,钴离子具有+2和+3的氧化态,因此为在电荷平衡框架中掺入Li提供了进一步的证据。红外光谱进一步证实了三个独立羟基的存在。磁化测量意味着在T = 25 K左右发生顺磁到反铁磁转变,在9-12 K左右发生第二次转变,其中铁磁分量低于该温度。通过热重分析和差示扫描量热法证明了该相的亚稳特性,在高于558°C的条件下,该相显示出两步分解为CoO,Co 3(PO 4)2和橄榄石型的LiCoPO 4释放出水和氧气。
更新日期:2017-08-29
中文翻译:

Co 11 Li [(OH)5 O] [(PO 3 OH)(PO 4)5 ],一种锂稳定的混合价氢氧化钴(II,III)磷酸盐骨架
一种新的亚稳态相,具有锂稳定的混合价钴(II,III)氢氧化钴磷酸盐骨架,Co 11.0(1) Li 1.0(2) [(OH)5 O] [(PO 3 OH)(PO 4)通过水热合成制备对应于简化组成Co 1.84(2) Li 0.16(3)(OH)PO 4的图5 ] 。因为其他相(例如Co 3(OH)2(PO 3 OH)2和橄榄石型LiCoPO 4)的pH依赖性形成竞争过程中,pH值5.0对于获得单相材料至关重要。尺寸为15μm×30μm的晶体表现出独特的细长三角锥形态,具有层状精细结构。粉末X射线衍射实验表明,该相与天然磷酸盐矿物holtedahlite和satterlyite是同构的,并且在三角空间群P 31 m(a = 11.2533(4)Å,c = 4.9940(2)Å,V = 547.70(3)3,Z = 1)。三维网络结构的特征在于部分被Li取代的八面体[M 2 O 8(OH)](M = Co,Li)二聚体单元,其形成沿[001]方向延伸的双链并通过[PO 4 ]和[PO 3(OH)]四面体连接。因为没有无锂的P 31 m型Co 2(OH)PO 4可以准备阶段,可以假设锂离子对于稳定框架至关重要。Co L边缘X射线吸收光谱表明,钴离子具有+2和+3的氧化态,因此为在电荷平衡框架中掺入Li提供了进一步的证据。红外光谱进一步证实了三个独立羟基的存在。磁化测量意味着在T = 25 K左右发生顺磁到反铁磁转变,在9-12 K左右发生第二次转变,其中铁磁分量低于该温度。通过热重分析和差示扫描量热法证明了该相的亚稳特性,在高于558°C的条件下,该相显示出两步分解为CoO,Co 3(PO 4)2和橄榄石型的LiCoPO 4释放出水和氧气。































 京公网安备 11010802027423号
京公网安备 11010802027423号