当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-Term Uptake of Phenol-Water Vapor Follows Similar Sigmoid Kinetics on Prehydrated Organic Matter- and Clay-Rich Soil Sorbents
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.est.7b01558 Mikhail Borisover 1 , Nadezhda Bukhanovsky 1 , Marcos Lado 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.est.7b01558 Mikhail Borisover 1 , Nadezhda Bukhanovsky 1 , Marcos Lado 2
Affiliation
|
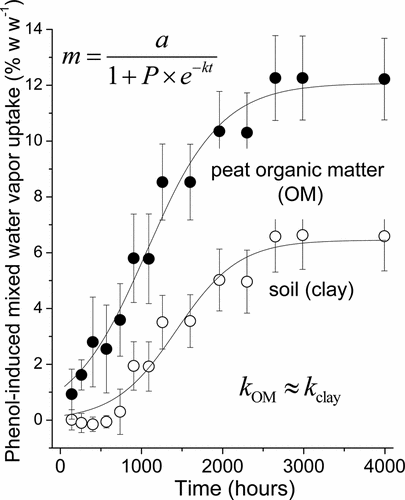
Typical experimental time frames allowed for equilibrating water-organic vapors with soil sorbents might lead to overlooking slow chemical reactions finally controlling a thermodynamically stable state. In this work, long-term gravimetric examination of kinetics covering about 4000 h was performed for phenol-water vapor interacting with four materials pre-equilibrated at three levels of air relative humidity (RHs 52, 73, and 92%). The four contrasting sorbents included an organic matter (OM)-rich peat soil, an OM-poor clay soil, a hydrophilic Aldrich humic acid salt, and water-insoluble leonardite. Monitoring phenol-water vapor interactions with the prehydrated sorbents, as compared with the sorbent samples in phenol-free atmosphere at the same RH, showed, for the first time, a sigmoid kinetics of phenol-induced mass uptake typical for second-order autocatalytic reactions. The apparent rate constants were similar for all the sorbents, RHs and phenol activities studied. A significant part of sorbed phenol resisted extraction, which was attributed to its abiotic oxidative coupling. Phenol uptake by peat and clay soils was also associated with a significant enhancement of water retention. The delayed development of the sigmoidal kinetics in phenol-water uptake demonstrates that long-run abiotic interactions of water–organic vapor with soil may be overlooked, based on short-term examination.
中文翻译:

苯酚-水蒸气的长期吸收对预水合的有机物和富含粘土的土壤吸收剂具有相似的乙状结肠动力学。
典型的实验时间框架允许用土壤吸附剂平衡水有机蒸气,这可能会导致忽略缓慢的化学反应,最终控制热力学稳定状态。在这项工作中,对酚-水蒸气与四种在空气相对湿度(RH 52、73和92%)条件下已预先平衡的物质相互作用的酚水蒸气进行了约4000小时的动力学动力学长期重量检查。四种形成对比的吸附剂包括富含有机物(OM)的泥炭土,缺乏OM的粘土,亲水性Aldrich腐植酸盐和水不溶性人造石。与在相同的相对湿度下在无苯酚气氛中的吸附剂样品相比,监测与预水合吸附剂的苯酚-水蒸气相互作用是第一次,二阶自催化反应中苯酚引起的质量吸收的S形动力学。所有研究的吸附剂,相对湿度和苯酚活性的表观速率常数均相似。吸附的苯酚有很大一部分抵抗了萃取,这归因于其非生物的氧化偶联作用。泥炭和黏土对苯酚的吸收也与保水能力的显着提高有关。乙二醛动力学在苯酚-水吸收中的延迟发展表明,根据短期检查,水-有机蒸气与土壤的长期非生物相互作用可能会被忽略。泥炭和黏土对苯酚的吸收也与保水能力的显着提高有关。乙二醛动力学在苯酚-水吸收中的延迟发展表明,根据短期检查,水-有机蒸气与土壤的长期非生物相互作用可能会被忽略。泥炭和黏土对苯酚的吸收也与保水能力的显着提高有关。乙二醛动力学在苯酚-水吸收中的延迟发展表明,根据短期检查,水-有机蒸气与土壤的长期非生物相互作用可能会被忽略。
更新日期:2017-08-29
中文翻译:

苯酚-水蒸气的长期吸收对预水合的有机物和富含粘土的土壤吸收剂具有相似的乙状结肠动力学。
典型的实验时间框架允许用土壤吸附剂平衡水有机蒸气,这可能会导致忽略缓慢的化学反应,最终控制热力学稳定状态。在这项工作中,对酚-水蒸气与四种在空气相对湿度(RH 52、73和92%)条件下已预先平衡的物质相互作用的酚水蒸气进行了约4000小时的动力学动力学长期重量检查。四种形成对比的吸附剂包括富含有机物(OM)的泥炭土,缺乏OM的粘土,亲水性Aldrich腐植酸盐和水不溶性人造石。与在相同的相对湿度下在无苯酚气氛中的吸附剂样品相比,监测与预水合吸附剂的苯酚-水蒸气相互作用是第一次,二阶自催化反应中苯酚引起的质量吸收的S形动力学。所有研究的吸附剂,相对湿度和苯酚活性的表观速率常数均相似。吸附的苯酚有很大一部分抵抗了萃取,这归因于其非生物的氧化偶联作用。泥炭和黏土对苯酚的吸收也与保水能力的显着提高有关。乙二醛动力学在苯酚-水吸收中的延迟发展表明,根据短期检查,水-有机蒸气与土壤的长期非生物相互作用可能会被忽略。泥炭和黏土对苯酚的吸收也与保水能力的显着提高有关。乙二醛动力学在苯酚-水吸收中的延迟发展表明,根据短期检查,水-有机蒸气与土壤的长期非生物相互作用可能会被忽略。泥炭和黏土对苯酚的吸收也与保水能力的显着提高有关。乙二醛动力学在苯酚-水吸收中的延迟发展表明,根据短期检查,水-有机蒸气与土壤的长期非生物相互作用可能会被忽略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号