当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion-Exchanged Zeolites Y for Selective Adsorption of Methyl Mercaptan from Natural Gas: Experimental Performance Evaluation and Computational Mechanism Explorations
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.iecr.7b01982
Xi Chen 1 , Ben-xian Shen 1 , Hui Sun 1 , Guo-xiong Zhan 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2017-08-28 00:00:00 , DOI: 10.1021/acs.iecr.7b01982
Xi Chen 1 , Ben-xian Shen 1 , Hui Sun 1 , Guo-xiong Zhan 1
Affiliation
|
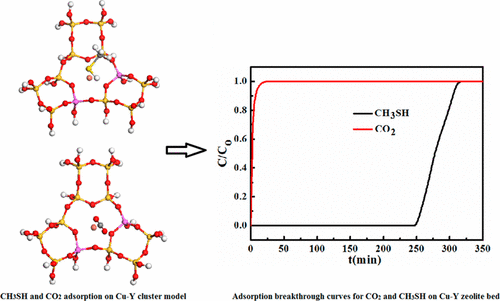
Zeolites Y were modified by ion-exchange method, and their structural properties were examined using N2 adsorption, Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and chemical composition analysis. The dynamic adsorption of methyl mercaptan (CH3SH) on different ion-exchanged zeolites Y was conducted on a fixed-bed adsorption column. The effects of gas hourly space velocity, operation temperature, and composition of the feed gas on the performance of CH3SH adsorption on ion-exchanged zeolites Y were studied carefully. Furthermore, the adsorption mechanism for CH3SH and CO2 adsorption on ion-exchanged zeolites was revealed by using density functional theory (DFT) calculation methods. Among all the ion-exchanged samples, Cu–Y holds the highest CH3SH breakthrough adsorption capacity, q, of up to 70 mg/g. When using natural gas containing 4% CO2 as the feed, q of Cu–Y was slightly reduced to 64 mg/g. The DFT calculation results indicate that the S–M bond is formed between CH3SH and Cu2+ during CH3SH adsorption, which benefits the adsorption of CH3SH on Cu–Y zeolite. Moreover, the DFT calculation suggests the weak Cu–O bonding interaction formed in the adsorption of CO2 on Cu–Y, the interaction of which releases much less energy compared with CH3SH adsorption. The CH3SH-saturated Cu–Y sample can be regenerated by thermal treatment under air atmosphere at 350 °C. After six adsorption–regeneration cycles, the regenerated Cu–Y shows q of 55 mg/g, which is 21.4% lower than that of the fresh sample.
中文翻译:

离子交换沸石Y对天然气中甲基硫醇的选择性吸附:实验性能评估和计算机理探索
通过离子交换法对沸石Y进行改性,并利用N 2吸附,傅立叶变换红外光谱,X射线衍射,X射线光电子能谱和化学组成分析来检查它们的结构性质。在固定床吸附柱上进行甲基硫醇(CH 3 SH)在不同离子交换沸石Y上的动态吸附。仔细研究了气体时空速度,操作温度和进料气组成对离子交换沸石Y吸附CH 3 SH性能的影响。此外,CH 3 SH和CO 2的吸附机理利用密度泛函理论(DFT)计算方法揭示了离子交换沸石的吸附。在所有离子交换样品中,Cu–Y拥有最高70 mg / g的最高CH 3 SH突破吸附容量q。当使用含4%CO 2的天然气作为进料时,Cu-Y的q值略微降低至64 mg / g。的DFT计算结果表明,在S-M键CH之间形成3 SH和Cu 2+ CH期间3 SH吸附,有利于CH的吸附3 SH对Cu-Y型沸石。此外,DFT计算表明在CO 2的吸附过程中形成了弱的Cu-O键相互作用在Cu-Y上,与CH 3 SH吸附相比,其相互作用释放的能量要少得多。CH 3 SH饱和的Cu-Y样品可以在350°C的空气气氛中通过热处理进行再生。经过六个吸附-再生循环后,再生的Cu-Y的定量q为55 mg / g,比新鲜样品低21.4%。
更新日期:2017-08-28
中文翻译:

离子交换沸石Y对天然气中甲基硫醇的选择性吸附:实验性能评估和计算机理探索
通过离子交换法对沸石Y进行改性,并利用N 2吸附,傅立叶变换红外光谱,X射线衍射,X射线光电子能谱和化学组成分析来检查它们的结构性质。在固定床吸附柱上进行甲基硫醇(CH 3 SH)在不同离子交换沸石Y上的动态吸附。仔细研究了气体时空速度,操作温度和进料气组成对离子交换沸石Y吸附CH 3 SH性能的影响。此外,CH 3 SH和CO 2的吸附机理利用密度泛函理论(DFT)计算方法揭示了离子交换沸石的吸附。在所有离子交换样品中,Cu–Y拥有最高70 mg / g的最高CH 3 SH突破吸附容量q。当使用含4%CO 2的天然气作为进料时,Cu-Y的q值略微降低至64 mg / g。的DFT计算结果表明,在S-M键CH之间形成3 SH和Cu 2+ CH期间3 SH吸附,有利于CH的吸附3 SH对Cu-Y型沸石。此外,DFT计算表明在CO 2的吸附过程中形成了弱的Cu-O键相互作用在Cu-Y上,与CH 3 SH吸附相比,其相互作用释放的能量要少得多。CH 3 SH饱和的Cu-Y样品可以在350°C的空气气氛中通过热处理进行再生。经过六个吸附-再生循环后,再生的Cu-Y的定量q为55 mg / g,比新鲜样品低21.4%。




































 京公网安备 11010802027423号
京公网安备 11010802027423号