Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-08-26 , DOI: 10.1016/j.bmcl.2017.08.053 Zhan Cai , Zichao Ding , Yumeng Hao , Tingjunhong Ni , Fei Xie , Jing Zhao , Ran Li , Shichong Yu , Ting Wang , Xiaoyun Chai , Yongsheng Jin , Yue Gao , Dazhi Zhang , Yuanying Jiang
|
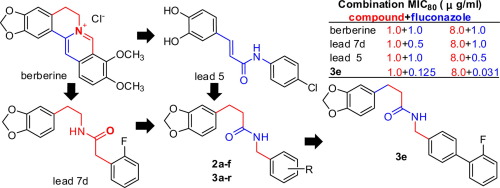
Based on our previous discovery and SAR study on the lead compounds 7d, 5 and berberine which can significantly enhance the susceptibility of fluconazole against fluconazole-resistant Candida albicans, a series of 3-(benzo[d][1,3]dioxol-5-yl)-N-(substituted benzyl)propanamides were designed, synthesized, and evaluated for their in vitro synergistic activity in combination with fluconazole. The series 2a–f were designed by replacing the amide moiety of the lead compound 7d with retro-amide moiety, and compounds 2a and 2b showed more activity than the lead 7d. Furthermore, introducing biphenyl moiety into series 2d–f afforded series 3a–r, most of which exhibited significantly superior activity to the series 2d–f. Especially, compound 3e, at a concentration of 1.0 µg/ml, can enhance the susceptibility of fluconazole against fluconazole-resistant Candida albicans from 128.0 µg/ml to 0.125–0.25 µg/ml. A clear SAR of the compounds is discussed.
中文翻译:

3-(苯并[ d ] [1,3]二恶酚-5-基)-N-苄基丙酰胺作为新型抗氟康唑耐药白色念珠菌的增效剂的设计,合成和SAR研究
基于我们先前对能显着增强氟康唑对耐氟康唑的白色念珠菌敏感性的先导化合物7d,5和小ber碱的发现和SAR研究,一系列3-(苯并[ d ] [1,3] dioxol-5 (氟基)-N-(取代的苄基)丙酰胺与氟康唑联合设计,合成并评估了它们的体外协同活性。2a – f系列的设计是通过用逆向酰胺部分取代先导化合物7d的酰胺部分,化合物2a和2b显示出比先导7d更高的活性。此外,将联苯部分引入系列2d– f提供了3a – r系列,其中大多数表现出明显优于2d – f系列的活性。特别是,化合物3e的浓度为1.0 µg / ml,可以使氟康唑对耐氟康唑的白色念珠菌的敏感性从128.0 µg / ml增加到0.125–0.25 µg / ml。讨论了化合物的清晰比吸收率。































 京公网安备 11010802027423号
京公网安备 11010802027423号