当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tin Oxides: Insights into Chemical States from a Nanoparticle Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acs.jpcc.7b05013 Charles Wright , Chaofan Zhang 1 , Mikko-Heikki Mikkelä , Erik Mårsell , Anders Mikkelsen , Stacey Sorensen , Olle Björneholm 1 , Maxim Tchaplyguine
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acs.jpcc.7b05013 Charles Wright , Chaofan Zhang 1 , Mikko-Heikki Mikkelä , Erik Mårsell , Anders Mikkelsen , Stacey Sorensen , Olle Björneholm 1 , Maxim Tchaplyguine
Affiliation
|
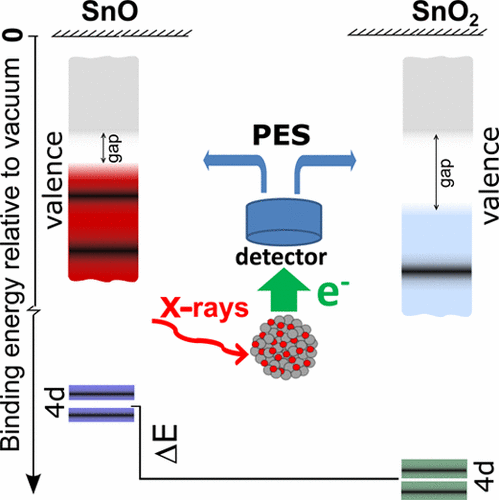
Tin oxides are semiconductor materials currently attracting close attention in electronics, photovoltaics, gas sensing, and catalysis. Depending on the tin oxidation state—Sn(IV), Sn(II), or intermediate—the corresponding oxide has either n- or p-type natural conductivity, ascribed to oxygen or metal deficiency in the lattice. Such crystalline imperfections severely complicate the task of establishing tin oxidation state, especially at nanoscale. In spite of the striking differences between SnO2 and SnO in their most fundamental properties, there have been enduring problems in identifying the oxide type. These problems were to a great extent caused by the controversy around the characteristic chemical shift, that is, the difference in electron binding energy of a certain core level in an oxide and its parent metal. Using in situ fabricated bare tin oxide nanoparticles, we have been able to resolve the controversy: Our photoelectron spectroscopic study on tin oxide nanoparticles shows that, in contrast to a common opinion of a close chemical shift for SnO2 and SnO, the shift value for tin(IV) oxide is, in fact, 3 times larger than that for tin(II) oxide. Moreover, our investigation of the nanoparticle valence electronic structure clarifies the question of why previously the identification of oxidation states encountered problems.
中文翻译:

氧化锡:纳米颗粒研究对化学状态的见解
氧化锡是目前在电子,光伏,气体传感和催化领域引起密切关注的半导体材料。根据锡的氧化态(Sn(IV),Sn(II)或中间体),相应的氧化物具有n型或p型自然电导率,归因于晶格中的氧或金属缺乏。这种晶体缺陷严重地使建立锡的氧化态的任务复杂化,尤其是在纳米级。尽管SnO 2之间存在显着差异在SnO和SnO的最基本特性中,在确定氧化物类型方面一直存在持久的问题。这些问题在很大程度上是由围绕特征化学位移的争议引起的,该争议是特征性化学位移,即氧化物及其母金属中某个核心能级的电子结合能的差异。使用原位制造的裸露的氧化锡纳米粒子,我们已经能够解决这一争议:我们对氧化锡纳米粒子的光电子光谱研究表明,与SnO 2的化学位移接近的普遍观点相反。实际上,氧化锡(IV)的位移值是氧化锡(II)的位移值的3倍。此外,我们对纳米价电子结构的研究澄清了为什么以前鉴定氧化态会遇到问题的问题。
更新日期:2017-08-26
中文翻译:

氧化锡:纳米颗粒研究对化学状态的见解
氧化锡是目前在电子,光伏,气体传感和催化领域引起密切关注的半导体材料。根据锡的氧化态(Sn(IV),Sn(II)或中间体),相应的氧化物具有n型或p型自然电导率,归因于晶格中的氧或金属缺乏。这种晶体缺陷严重地使建立锡的氧化态的任务复杂化,尤其是在纳米级。尽管SnO 2之间存在显着差异在SnO和SnO的最基本特性中,在确定氧化物类型方面一直存在持久的问题。这些问题在很大程度上是由围绕特征化学位移的争议引起的,该争议是特征性化学位移,即氧化物及其母金属中某个核心能级的电子结合能的差异。使用原位制造的裸露的氧化锡纳米粒子,我们已经能够解决这一争议:我们对氧化锡纳米粒子的光电子光谱研究表明,与SnO 2的化学位移接近的普遍观点相反。实际上,氧化锡(IV)的位移值是氧化锡(II)的位移值的3倍。此外,我们对纳米价电子结构的研究澄清了为什么以前鉴定氧化态会遇到问题的问题。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号