当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetraterpene Synthase Substrate and Product Specificity in the Green Microalga Botryococcus braunii Race L
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acschembio.7b00457 Hem R. Thapa 1 , Su Tang 1 , James C. Sacchettini 1, 2 , Timothy P. Devarenne 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1021/acschembio.7b00457 Hem R. Thapa 1 , Su Tang 1 , James C. Sacchettini 1, 2 , Timothy P. Devarenne 1
Affiliation
|
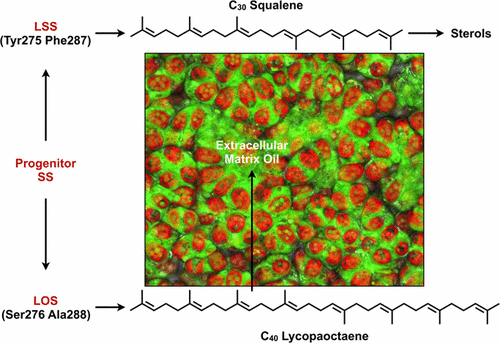
Recently, the biosynthetic pathway for lycopadiene, a C40 tetraterpenoid hydrocarbon, was deciphered from the L race of Botryococcus braunii, an alga that produces hydrocarbon oils capable of being converted into combustible fuels. The lycopadiene pathway is initiated by the squalene synthase (SS)-like enzyme lycopaoctaene synthase (LOS), which catalyzes the head-to-head condensation of two C20 geranylgeranyl diphosphate (GGPP) molecules to produce C40 lycopaoctaene. LOS shows unusual substrate promiscuity for SS or SS-like enzymes by utilizing C15 farnesyl diphosphate (FPP) and C20 phytyl diphosphate in addition to GGPP as substrates. These three substrates can be combined by LOS individually or in combinations to produce six different hydrocarbons of C30, C35, and C40 chain lengths. To understand LOS substrate and product specificity, rational mutagenesis experiments were conducted based on sequence alignment with several SS proteins as well as a structural comparison with the human SS (HSS) crystal structure. Characterization of the LOS mutants in vitro identified Ser276 and Ala288 in the LOS active site as key amino acids responsible for controlling substrate binding, and thus the promiscuity of this enzyme. Mutating these residues to those found in HSS largely converted LOS from lycopaoctaene production to C30 squalene production. Furthermore, these studies were confirmed in vivo by expressing LOS in E. coli cells metabolically engineered to produce high FPP and GGPP levels. These studies also offer insights into tetraterpene hydrocarbon metabolism in B. braunii and provide a foundation for engineering LOS for robust production of specific hydrocarbons of a desired chain length.
中文翻译:

四萜合酶底物和绿色的微藻Botryococcus braunii种族L中的产品特异性。
最近,番茄红素的生物合成途径是C 40四萜类碳氢化合物,它是从藻类Botryococcus braunii的L族中解密得到的,藻类产生的烃油能够转化为可燃燃料。番茄红素途径是由角鲨烯合酶(SS)样酶lycopaoctaene合酶(LOS)引发的,它催化两个C 20香叶基香叶基二磷酸(GGPP)分子的头对头缩合,生成C 40 lycopaoctaene。LOS通过利用C 15法呢基二磷酸(FPP)和C 20显示SS或SS类酶的底物混杂现象除了GGPP以外,植酸二磷酸为底物。可以通过LOS单独或组合使用这三种底物,以生产链长为C 30,C 35和C 40的六种不同的烃。为了了解LOS底物和产物的特异性,基于与几种SS蛋白的序列比对以及与人SS(HSS)晶体结构的结构比较,进行了合理的诱变实验。LOS突变体的体外表征在LOS活性位点鉴定出Ser276和Ala288是负责控制底物结合并因此控制该酶混杂的关键氨基酸。将这些残基突变为HSS中发现的残基,可将LOS从番木辛烯生产转变为C 30角鲨烯生产。此外,这些研究在体内经过代谢工程化以产生高FPP和GGPP水平的大肠杆菌细胞中表达LOS而得到证实。这些研究还提供了对布鲁氏双歧杆菌中四萜碳氢化合物代谢的见识,并为工程LOS的基础奠定了基础,以稳定地生产所需链长的特定碳氢化合物。
更新日期:2017-08-26
中文翻译:

四萜合酶底物和绿色的微藻Botryococcus braunii种族L中的产品特异性。
最近,番茄红素的生物合成途径是C 40四萜类碳氢化合物,它是从藻类Botryococcus braunii的L族中解密得到的,藻类产生的烃油能够转化为可燃燃料。番茄红素途径是由角鲨烯合酶(SS)样酶lycopaoctaene合酶(LOS)引发的,它催化两个C 20香叶基香叶基二磷酸(GGPP)分子的头对头缩合,生成C 40 lycopaoctaene。LOS通过利用C 15法呢基二磷酸(FPP)和C 20显示SS或SS类酶的底物混杂现象除了GGPP以外,植酸二磷酸为底物。可以通过LOS单独或组合使用这三种底物,以生产链长为C 30,C 35和C 40的六种不同的烃。为了了解LOS底物和产物的特异性,基于与几种SS蛋白的序列比对以及与人SS(HSS)晶体结构的结构比较,进行了合理的诱变实验。LOS突变体的体外表征在LOS活性位点鉴定出Ser276和Ala288是负责控制底物结合并因此控制该酶混杂的关键氨基酸。将这些残基突变为HSS中发现的残基,可将LOS从番木辛烯生产转变为C 30角鲨烯生产。此外,这些研究在体内经过代谢工程化以产生高FPP和GGPP水平的大肠杆菌细胞中表达LOS而得到证实。这些研究还提供了对布鲁氏双歧杆菌中四萜碳氢化合物代谢的见识,并为工程LOS的基础奠定了基础,以稳定地生产所需链长的特定碳氢化合物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号