当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential [1 + 4]- and [2 + 3]-Annulation of Prop-2-ynylsulfonium Salts: Access to Hexahydropyrrolo[3,2-b]indoles
Organic Letters ( IF 4.9 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1021/acs.orglett.7b02298 Penghao Jia 1 , Qinglong Zhang 1 , Qima Ou 1 , You Huang 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1021/acs.orglett.7b02298 Penghao Jia 1 , Qinglong Zhang 1 , Qima Ou 1 , You Huang 1, 2
Affiliation
|
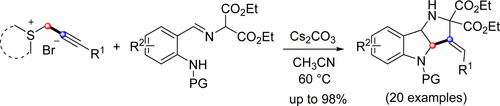
A powerful sequential [1 + 4]- and [2 + 3]-annulation has been developed using prop-2-ynylsulfonium salts and sulfonyl-protected o-amino aromatic aldimines, affording a series of hexahydropyrrolo[3,2-b]indoles in high yields. Prop-2-ynylsulfonium salts act as C2 synthons in the reaction, and fused rings containing two five-membered azaheterocycles can be constructed in a single operation with readily accessible starting materials.
中文翻译:

顺序的[1 + 4]-和[2 + 3]-环丙基-2-炔基Salt盐:进入六氢吡咯并[3,2- b ]吲哚
使用丙-2-炔基salts盐和磺酰基保护的邻氨基芳族亚胺开发了有力的顺序[1 + 4]-和[2 + 3]环化反应,提供了一系列的六氢吡咯并[3,2- b ]吲哚高产。丙-2-炔基salts盐在反应中充当C 2合成子,并且可以在一次操作中使用易于获得的起始原料构建包含两个五元氮杂杂环的稠合环。
更新日期:2017-08-22
中文翻译:

顺序的[1 + 4]-和[2 + 3]-环丙基-2-炔基Salt盐:进入六氢吡咯并[3,2- b ]吲哚
使用丙-2-炔基salts盐和磺酰基保护的邻氨基芳族亚胺开发了有力的顺序[1 + 4]-和[2 + 3]环化反应,提供了一系列的六氢吡咯并[3,2- b ]吲哚高产。丙-2-炔基salts盐在反应中充当C 2合成子,并且可以在一次操作中使用易于获得的起始原料构建包含两个五元氮杂杂环的稠合环。






























 京公网安备 11010802027423号
京公网安备 11010802027423号