当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P-Chirogenic Xantphos Ligands and Related Ether Diphosphines: Synthesis and Application in Rhodium-Catalyzed Asymmetric Hydrogenation
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1021/acscatal.7b01260 Jens Holz 1 , Katharina Rumpel 1 , Anke Spannenberg 1 , Rocco Paciello 2 , Haijun Jiao 1 , Armin Börner 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1021/acscatal.7b01260 Jens Holz 1 , Katharina Rumpel 1 , Anke Spannenberg 1 , Rocco Paciello 2 , Haijun Jiao 1 , Armin Börner 1, 3
Affiliation
|
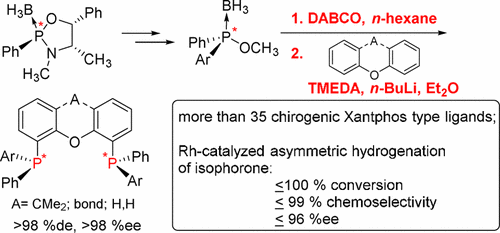
A series of P-chirogenic Xantphos ligands and related diaryl ether diphosphines have been synthesized by a modification of the well-established Jugé method. The approach consists of the in situ deboranation of the chiral ephedrine-based phosphinite before the P–C coupling takes place. The stereochemical integrity of the stereocenters of the diphosphines during synthesis, long-time storage, and catalytic application was evaluated. In the rhodium-catalyzed asymmetric hydrogenation of isophorone as a model substrate for industrially relevant prostereogenic enones with some of the diphosphines, almost complete conversion, high chemoselectivity, and 96% ee were achieved.
中文翻译:

P-深生黄药磷配体及相关醚二膦类化合物的合成及在铑催化的不对称加氢中的应用
通过完善的Jugé方法的改进,已经合成了一系列的P-手性黄原磷配体和相关的二芳基醚二膦。该方法包括在进行P-C偶联之前,对基于手性麻黄碱的次膦酸酯进行原位脱硼化处理。评价了在合成,长时间存储和催化应用过程中二膦的立体中心的立体化学完整性。在铑催化的异佛尔酮的不对称氢化反应中,作为工业上与之相关的具有二膦基化合物的孕甾烯酮的模型底物,可以实现几乎完全的转化,高的化学选择性和96%的ee。
更新日期:2017-08-19
中文翻译:

P-深生黄药磷配体及相关醚二膦类化合物的合成及在铑催化的不对称加氢中的应用
通过完善的Jugé方法的改进,已经合成了一系列的P-手性黄原磷配体和相关的二芳基醚二膦。该方法包括在进行P-C偶联之前,对基于手性麻黄碱的次膦酸酯进行原位脱硼化处理。评价了在合成,长时间存储和催化应用过程中二膦的立体中心的立体化学完整性。在铑催化的异佛尔酮的不对称氢化反应中,作为工业上与之相关的具有二膦基化合物的孕甾烯酮的模型底物,可以实现几乎完全的转化,高的化学选择性和96%的ee。































 京公网安备 11010802027423号
京公网安备 11010802027423号