当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study on M1/POM Single-Atom Catalysts (M = Cu, Zn, Ag, and Au; POM = [PW12O40]3–): Metal–Support Interactions and Catalytic Cycle for Alkene Epoxidation
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01480 Chun-Guang Liu 1 , Meng-Xu Jiang 1 , Zhong-Min Su 1, 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01480 Chun-Guang Liu 1 , Meng-Xu Jiang 1 , Zhong-Min Su 1, 2
Affiliation
|
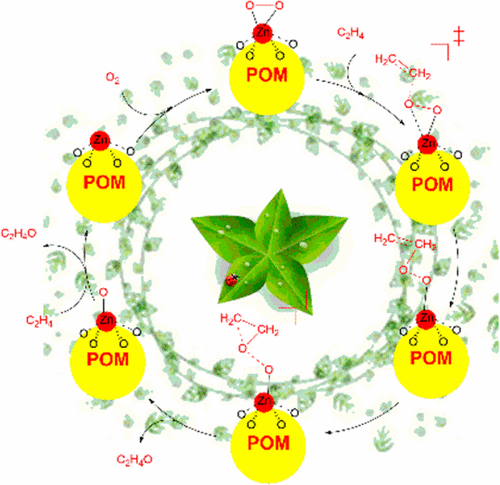
Geometrical structures, metal–support interactions, and infrared (IR) spectroscopy of a series of M1/POM (M = Cu, Zn, Ag, and Au; POM = [PW12O40]3–) single-atom catalysts (SACs), and catalytic cycle for alkene epoxidation catalyzed by M1/POM SACs were studied using density functional theory (DFT) calculations. The calculations demonstrate that the most probable anchoring sties for the isolated single atoms studied here in the M1/POM SACs are the fourfold hollow sites on the surface of POM support. The bonding interaction between single metal atom and surface of POM support comes from the molecular orbitals with a mixture of d atomic orbital of metal and 2p group orbital of surface oxygen atoms of POM cage. The calculated adsorption energy of isolated metal atoms in these M1/POM SACs indicates that the early transition metals (Cu and Zn) have high thermal stability. The DFT-derived IR spectra show that the four characteristic peaks of free Keggin-type POM structure split into six because of introduction of isolated metal atom. Compared with other metal atoms, the Zn1/POM SAC has the high reactivity for activity of dioxygen molecule, because the dioxygen moiety in Zn1/POM SAC displays O2–· radical feature with [POM4–·Zn2+O2–·]3– configuration. Finally, a catalytic cycle for ethylene epoxidation by O2 catalyzed by Zn1/POM SAC was proposed based on our DFT calculations. Supported noble-metal SACs are among the most important catalysts currently. However, noble metals are expensive and of limited supply. Development of non-noble-metal SACs is of essential importance. Therefore, the reported Zn1/POM SAC would be very useful to guide the search for SACs into non-noble metals.
中文翻译:

M 1 / POM单原子催化剂(M = Cu,Zn,Ag和Au; POM = [PW 12 O 40 ] 3–)的计算研究:烯烃环氧化的金属-载体相互作用和催化循环
一系列M 1 / POM(M = Cu,Zn,Ag和Au; POM = [PW 12 O 40 ] 3–)单原子催化剂的几何结构,金属与金属的相互作用以及红外(IR)光谱SACs,并使用密度泛函理论(DFT)计算研究了M 1 / POM SACs催化烯烃环氧化的催化循环。计算表明,M 1中研究的单个单原子最可能的锚固性/ POM SAC是POM支架表面上的四个空心位置。单个金属原子与POM载体表面之间的键相互作用来自分子轨道,该分子轨道具有金属的d原子轨道和POM笼的表面氧原子的2p基团轨道的混合物。计算出的这些M 1 / POM SAC中孤立的金属原子的吸附能表明,早期过渡金属(Cu和Zn)具有很高的热稳定性。DFT衍生的红外光谱表明,由于引入了孤立的金属原子,自由的Keggin型POM结构的四个特征峰分为六个。与其他金属原子相比,Zn 1 / POM SAC对双氧分子的活性具有较高的反应性,因为Zn 1中的双氧部分/ POM SAC显示O 2 – ·具有[POM 4– ·Zn 2+ O 2 – ·] 3–配置的自由基特征。最后,基于我们的DFT计算,提出了Zn 1 / POM SAC催化O 2催化乙烯环氧化的催化循环。负载的贵金属SAC是目前最重要的催化剂。但是,贵金属价格昂贵并且供应有限。非贵金属SAC的开发至关重要。因此,所报道的Zn 1 / POM SAC对于指导将SAC搜寻成非贵金属将是非常有用的。
更新日期:2017-08-18
中文翻译:

M 1 / POM单原子催化剂(M = Cu,Zn,Ag和Au; POM = [PW 12 O 40 ] 3–)的计算研究:烯烃环氧化的金属-载体相互作用和催化循环
一系列M 1 / POM(M = Cu,Zn,Ag和Au; POM = [PW 12 O 40 ] 3–)单原子催化剂的几何结构,金属与金属的相互作用以及红外(IR)光谱SACs,并使用密度泛函理论(DFT)计算研究了M 1 / POM SACs催化烯烃环氧化的催化循环。计算表明,M 1中研究的单个单原子最可能的锚固性/ POM SAC是POM支架表面上的四个空心位置。单个金属原子与POM载体表面之间的键相互作用来自分子轨道,该分子轨道具有金属的d原子轨道和POM笼的表面氧原子的2p基团轨道的混合物。计算出的这些M 1 / POM SAC中孤立的金属原子的吸附能表明,早期过渡金属(Cu和Zn)具有很高的热稳定性。DFT衍生的红外光谱表明,由于引入了孤立的金属原子,自由的Keggin型POM结构的四个特征峰分为六个。与其他金属原子相比,Zn 1 / POM SAC对双氧分子的活性具有较高的反应性,因为Zn 1中的双氧部分/ POM SAC显示O 2 – ·具有[POM 4– ·Zn 2+ O 2 – ·] 3–配置的自由基特征。最后,基于我们的DFT计算,提出了Zn 1 / POM SAC催化O 2催化乙烯环氧化的催化循环。负载的贵金属SAC是目前最重要的催化剂。但是,贵金属价格昂贵并且供应有限。非贵金属SAC的开发至关重要。因此,所报道的Zn 1 / POM SAC对于指导将SAC搜寻成非贵金属将是非常有用的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号