当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intermolecular Aryne Ene Reaction of Hantzsch Esters: Stable Covalent Ene Adducts from a 1,4-Dihydropyridine Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acs.orglett.7b02272 Piera Trinchera 1 , Weitao Sun 1 , Jane E. Smith 1 , David Palomas 1 , Rachel Crespo-Otero 1 , Christopher R. Jones 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acs.orglett.7b02272 Piera Trinchera 1 , Weitao Sun 1 , Jane E. Smith 1 , David Palomas 1 , Rachel Crespo-Otero 1 , Christopher R. Jones 1
Affiliation
|
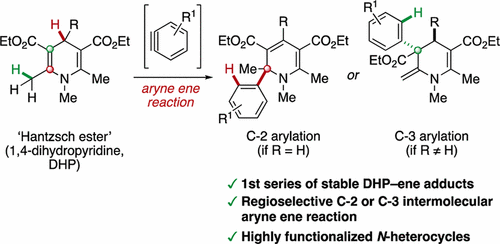
The reaction of arynes with 1,4-dihydropyridines affords 2-aryl-1,2-dihydropyridines or 2-methylene-3-aryl-1,2,3,4-tetrahydropyridines via a regioselective C-2 or C-3 arylation. These compounds are the first series of isolable and bench-stable covalent ene adducts formed between dihydropyridines and unsaturated substrates. Experimental studies and DFT calculations provide mechanistic support for a concerted intermolecular aryne ene process, which may have implications for NAD(P)H model reactions.
中文翻译:

Hantzsch酯的分子间Aryne烯反应:来自1,4-二氢吡啶反应的稳定的共价烯加合物
芳烃与1,4-二氢吡啶的反应通过区域选择性的C-2或C-3芳基化反应得到2-芳基-1,2-二氢吡啶或2-亚甲基-3-芳基-1,2,3,4-四氢吡啶。这些化合物是在二氢吡啶和不饱和底物之间形成的第一组可分离且稳定的共价烯加合物。实验研究和DFT计算为协同的分子间亚芳烃工艺提供了机械支持,这可能对NAD(P)H模型反应有影响。
更新日期:2017-08-17
中文翻译:

Hantzsch酯的分子间Aryne烯反应:来自1,4-二氢吡啶反应的稳定的共价烯加合物
芳烃与1,4-二氢吡啶的反应通过区域选择性的C-2或C-3芳基化反应得到2-芳基-1,2-二氢吡啶或2-亚甲基-3-芳基-1,2,3,4-四氢吡啶。这些化合物是在二氢吡啶和不饱和底物之间形成的第一组可分离且稳定的共价烯加合物。实验研究和DFT计算为协同的分子间亚芳烃工艺提供了机械支持,这可能对NAD(P)H模型反应有影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号