当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Label-Free Homogeneous Electrochemical Sensing Platform for Protein Kinase Assay Based on Carboxypeptidase Y-Assisted Peptide Cleavage and Vertically Ordered Mesoporous Silica Films
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acs.analchem.7b01739 Jinquan Liu 1 , Hong Cheng 1 , Dinggeng He 1 , Xiaoxiao He 1 , Kemin Wang 1 , Qiaoqiao Liu 1 , Shuaiqi Zhao 1 , Xudong Yang 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-08-17 00:00:00 , DOI: 10.1021/acs.analchem.7b01739 Jinquan Liu 1 , Hong Cheng 1 , Dinggeng He 1 , Xiaoxiao He 1 , Kemin Wang 1 , Qiaoqiao Liu 1 , Shuaiqi Zhao 1 , Xudong Yang 1
Affiliation
|
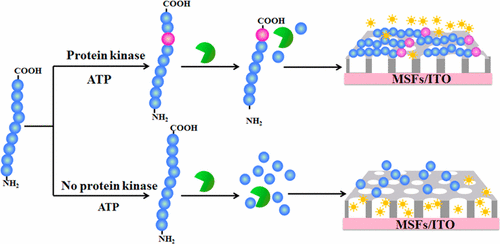
Presented herein is a simple, robust, and label-free homogeneous electrochemical sensing platform constructed for the detection of protein kinase activity and inhibition by integration of carboxypeptidase Y (CPY)-assisted peptide cleavage reaction and vertically ordered mesoporous silica films (MSFs). In this sensing platform, the substrate peptide composed of kinase-specific recognized sequence and multiple positively charged arginine (R) residues was ingeniously designed. In the presence of protein kinase, the substrate peptide was phosphorylated and then immediately resisted CPY cleavage. The phosphorylated peptide could be effectively adsorbed on the negatively charged surface of MSFs modified indium–tin oxide (ITO) electrode (MSFs/ITO) by noncovalent electrostatic attraction. The adsorbed peptide was subsequently used as a hamper to prevent the diffusion of electroactive probe (FcMeOH) to the electrode surface through the vertically aligned nanopores, resulting in a detectable reduction of electrochemical signal. As demonstrated for the feasibility and universality of the sensing platform, both protein kinase A (PKA) and casein kinase II (CK2) were selected as the models, and the detection limits were determined to be 0.083 and 0.095 UmL–1, respectively. This sensing platform had the merits of simplicity, easy manipulation, and improved phosphorylation and cleavage efficiency, which benefited from homogeneous solution reactions without sophisticated modification or immobilization procedures. In addition, given the key role of inhibition and protein kinase activity detection in cell lysates, this proposed sensing platform showed great potential in kinase-related bioanalysis and clinical biomedicine.
中文翻译:

基于羧肽酶Y辅助肽切割和垂直有序介孔硅胶膜的蛋白激酶测定的无标签均质电化学传感平台。
本文介绍的是一种简单,耐用且无标签的均质电化学传感平台,该平台构建用于通过整合羧肽酶Y(CPY)辅助的肽裂解反应和垂直排列的介孔二氧化硅膜(MSF)来检测蛋白激酶活性和抑制作用。在该传感平台上,巧妙地设计了由激酶特异性识别序列和多个带正电荷的精氨酸(R)残基组成的底物肽。在存在蛋白激酶的情况下,底物肽被磷酸化,然后立即抵抗CPY裂解。磷酸化的肽可以通过非共价静电吸引作用有效地吸附在MSF修饰的铟锡氧化物(ITO)电极(MSFs / ITO)的带负电荷的表面上。随后将吸附的肽用作阻碍物,以防止电活性探针(FcMeOH)通过垂直排列的纳米孔扩散到电极表面,从而导致电化学信号的可检测到的减少。如证明了该传感平台的可行性和通用性,同时选择了蛋白激酶A(PKA)和酪蛋白激酶II(CK2)作为模型,检测限分别为0.083和0.095 UmL。分别为–1。该传感平台具有简单,易于操作,提高的磷酸化和裂解效率等优点,这得益于均相溶液反应,无需复杂的修饰或固定步骤。此外,鉴于抑制作用和蛋白裂解酶活性检测在细胞裂解物中的关键作用,该拟议的传感平台在激酶相关的生物分析和临床生物医学中显示出巨大的潜力。
更新日期:2017-08-17
中文翻译:

基于羧肽酶Y辅助肽切割和垂直有序介孔硅胶膜的蛋白激酶测定的无标签均质电化学传感平台。
本文介绍的是一种简单,耐用且无标签的均质电化学传感平台,该平台构建用于通过整合羧肽酶Y(CPY)辅助的肽裂解反应和垂直排列的介孔二氧化硅膜(MSF)来检测蛋白激酶活性和抑制作用。在该传感平台上,巧妙地设计了由激酶特异性识别序列和多个带正电荷的精氨酸(R)残基组成的底物肽。在存在蛋白激酶的情况下,底物肽被磷酸化,然后立即抵抗CPY裂解。磷酸化的肽可以通过非共价静电吸引作用有效地吸附在MSF修饰的铟锡氧化物(ITO)电极(MSFs / ITO)的带负电荷的表面上。随后将吸附的肽用作阻碍物,以防止电活性探针(FcMeOH)通过垂直排列的纳米孔扩散到电极表面,从而导致电化学信号的可检测到的减少。如证明了该传感平台的可行性和通用性,同时选择了蛋白激酶A(PKA)和酪蛋白激酶II(CK2)作为模型,检测限分别为0.083和0.095 UmL。分别为–1。该传感平台具有简单,易于操作,提高的磷酸化和裂解效率等优点,这得益于均相溶液反应,无需复杂的修饰或固定步骤。此外,鉴于抑制作用和蛋白裂解酶活性检测在细胞裂解物中的关键作用,该拟议的传感平台在激酶相关的生物分析和临床生物医学中显示出巨大的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号