JAMA Oncology ( IF 22.5 ) Pub Date : 2017-08-17 , DOI: 10.1001/jamaoncol.2017.2411 Thomas Powles 1 , Peter H O'Donnell 2 , Christophe Massard 3 , Hendrik-Tobias Arkenau 4 , Terence W Friedlander 5 , Christopher J Hoimes 6 , Jae Lyun Lee 7 , Michael Ong 8 , Srikala S Sridhar 9 , Nicholas J Vogelzang 10 , Mayer N Fishman 11 , Jingsong Zhang 11 , Sandy Srinivas 12 , Jigar Parikh 13 , Joyce Antal 14 , Xiaoping Jin 14 , Ashok K Gupta 14 , Yong Ben 15 , Noah M Hahn 16
|
Importance The data reported herein were accepted for assessment by the US Food and Drug Administration for Biologics License Application under priority review to establish the clinical benefit of durvalumab as second-line therapy for locally advanced or metastatic urothelial carcinoma (UC), resulting in its recent US approval.
Objective To report a planned update of the safety and efficacy of durvalumab in patients with locally advanced/metastatic UC.
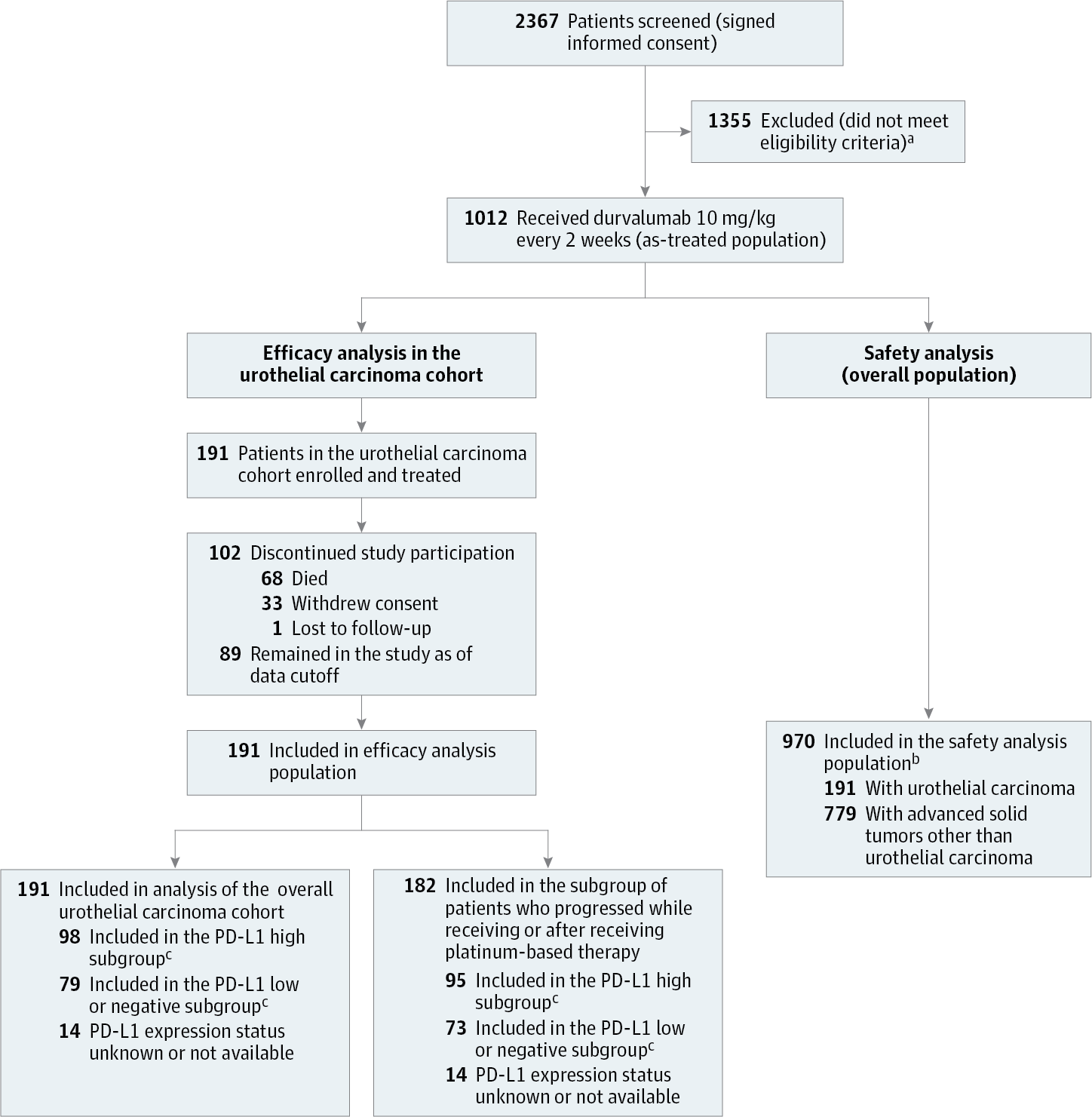
Design, Setting, and Participants This is an ongoing phase 1/2 open-label study of 191 adult patients with histologically or cytologically confirmed locally advanced/metastatic UC whose disease had progressed on, were ineligible for, or refused prior chemotherapy from 60 sites in 9 countries as reported herein.
Intervention Patients were administered durvalumab intravenous infusion, 10 mg/kg every 2 weeks, for up to 12 months or until progression, starting another anticancer therapy, or unacceptable toxic effects.
Main Outcomes and Measures Primary end points were safety and confirmed objective response rate (ORR) per blinded independent central review (Response Evaluation Criteria In Solid Tumors [RECIST], version 1.1).
Results A total of 191 patients with UC had received treatment. As of October 24, 2016 (90-day update), the median follow-up was 5.78 months (range, 0.4-25.9 months). The median age of patients was 67.0 years and most were male (136 [71.2%]) and white (123 [71.1%]). All patients had stage 4 disease, and 190 (99.5%) had prior anticancer therapy (182 [95.3%] postplatinum). The ORR was 17.8% (34 of 191; 95% CI, 12.7%-24.0%), including 7 complete responses. Responses were early (median time to response, 1.41 months), durable (median duration of response not reached), and observed regardless of programmed cell death ligand-1 (PD-L1) expression (ORR, 27.6% [n = 27; 95% CI, 19.0%-37.5%] and 5.1% [n = 4; 95% CI, 1.4%-12.5%] in patients with high and low or negative expression of PD-L1, respectively). Median progression-free survival and overall survival were 1.5 months (95% CI, 1.4-1.9 months) and 18.2 months (95% CI, 8.1 months to not estimable), respectively; the 1-year overall survival rate was 55% (95% CI, 44%-65%), as estimated by Kaplan-Meier method. Grade 3/4 treatment-related adverse events (AEs) occurred in 13 patients (6.8%); grade 3/4 immune-mediated AEs occurred in 4 patients (2.1%); and treatment-related AEs led to discontinuation of 3 patients (1.6%), 2 of whom had immune-mediated AEs that led to death (autoimmune hepatitis and pneumonitis).
Conclusions and Relevance Durvalumab, 10 mg/kg every 2 weeks, demonstrates favorable clinical activity and an encouraging and manageable safety profile in patients with locally advanced/metastatic UC.
Trial Registration clinicaltrials.gov Identifier: NCT01693562
中文翻译:

Durvalumab 在局部晚期或转移性尿路上皮癌中的疗效和安全性来自 1/2 期开放标签研究的更新结果
重要性 本文报告的数据已被美国食品和药物管理局生物制品许可申请接受评估,优先审查以确定 durvalumab 作为局部晚期或转移性尿路上皮癌 (UC) 的二线治疗的临床益处,导致其最近美国批准。
目的 报告计划更新 durvalumab 在局部晚期/转移性 UC 患者中的安全性和有效性。
设计、设置和参与者 这是一项正在进行的 1/2 期开放标签研究,对 191 名经组织学或细胞学证实的局部晚期/转移性 UC 成年患者进行了研究,这些患者的疾病已经在 60 个地点进展、不适合或拒绝先前的化疗。本文报道的 9 个国家。
干预 患者接受 durvalumab 静脉输注,每 2 周 10 mg/kg,持续长达 12 个月或直至疾病进展、开始另一种抗癌治疗或出现不可接受的毒性作用。
主要结果和措施 主要终点是安全性和每个盲法独立中央审查确认的客观反应率 (ORR)(实体瘤反应评估标准 [RECIST],版本 1.1)。
结果 共有 191 名 UC 患者接受了治疗。截至 2016 年 10 月 24 日(90 天更新),中位随访时间为 5.78 个月(范围 0.4-25.9 个月)。患者的中位年龄为 67.0 岁,大多数为男性 (136 [71.2%]) 和白人 (123 [71.1%])。所有患者均患有 4 期疾病,190 名 (99.5%) 曾接受过抗癌治疗(182 名 [95.3%] 接受铂后治疗)。ORR 为 17.8%(191 个中的 34 个;95% CI,12.7%-24.0%),包括 7 个完全缓解。反应是早期的(中位反应时间,1.41 个月),持久的(未达到中位反应持续时间),并且无论程序性细胞死亡配体 1 (PD-L1) 表达如何都可以观察到(ORR,27.6% [n = 27; 95 % CI, 19.0%-37.5%] 和 5.1% [n = 4; 95% CI, 1.4%-12.5%] 分别在 PD-L1 高表达和低表达或阴性表达的患者中)。中位无进展生存期和总生存期为 1。分别为 5 个月(95% CI,1.4-1.9 个月)和 18.2 个月(95% CI,8.1 个月至不可估计);根据 Kaplan-Meier 方法估计,1 年总生存率为 55%(95% CI,44%-65%)。13 名患者 (6.8%) 发生 3/4 级治疗相关不良事件 (AE);4 名患者 (2.1%) 发生了 3/4 级免疫介导的 AE;和治疗相关的 AE 导致 3 名患者(1.6%)停药,其中 2 名患者出现导致死亡的免疫介导 AE(自身免疫性肝炎和肺炎)。
结论和相关性 Durvalumab,每 2 周 10 mg/kg,在局部晚期/转移性 UC 患者中表现出良好的临床活性和令人鼓舞且可控的安全性。
试验注册 临床试验.gov 标识符:NCT01693562




















































 京公网安备 11010802027423号
京公网安备 11010802027423号