当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A first-principles study on the B5O5+/0 and B5O5− clusters: The boron oxide analogs of C6H5+/0 and CH3Cl
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-08-12 13:28:19 , DOI: 10.1063/1.4928282 Wen-Juan Tian 1 , Xue-Rui You 1 , Da-Zhi Li 2 , Ting Ou 1 , Qiang Chen 1 , Hua-Jin Zhai 1, 3 , Si-Dian Li 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-08-12 13:28:19 , DOI: 10.1063/1.4928282 Wen-Juan Tian 1 , Xue-Rui You 1 , Da-Zhi Li 2 , Ting Ou 1 , Qiang Chen 1 , Hua-Jin Zhai 1, 3 , Si-Dian Li 1
Affiliation
|
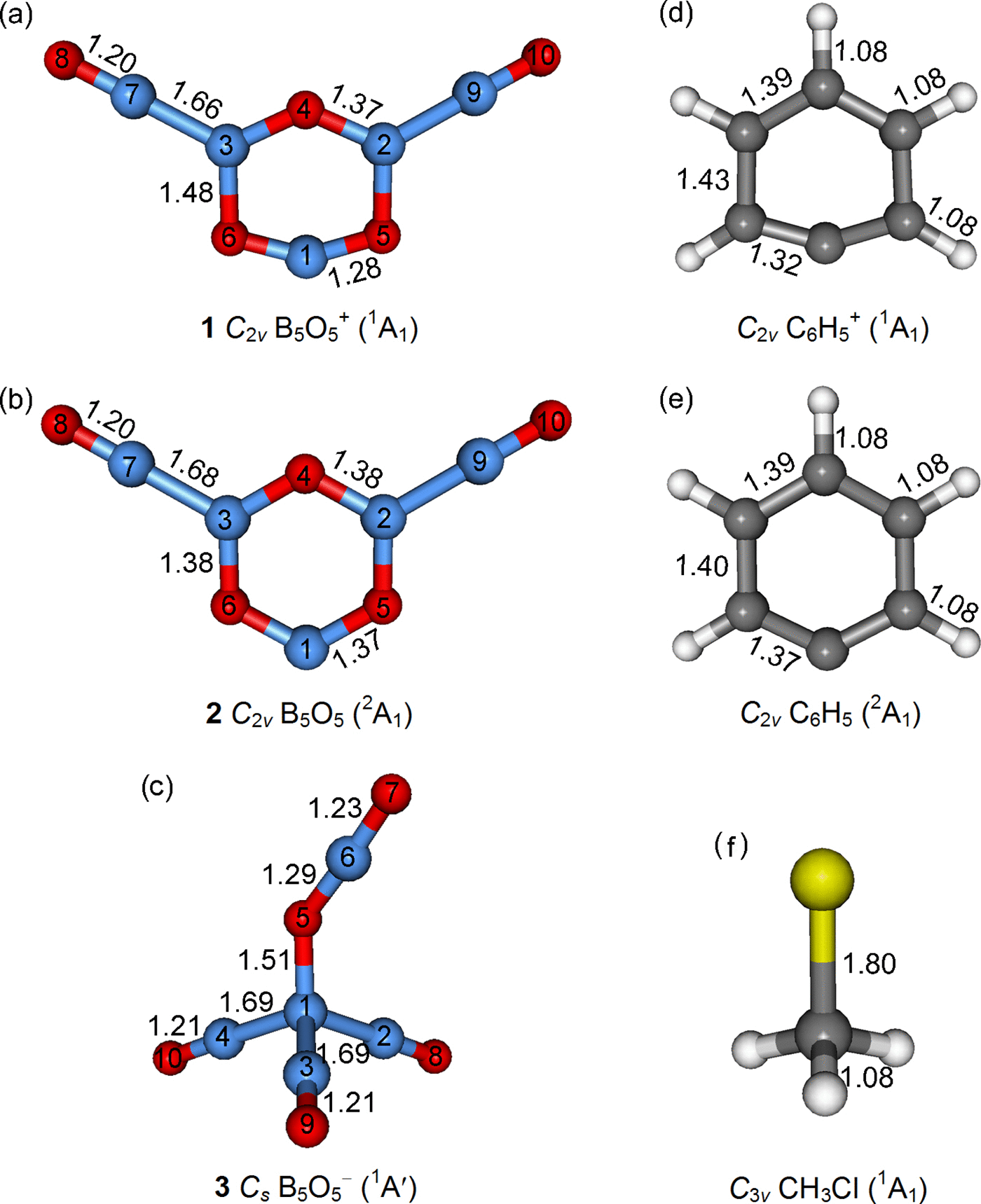
The concept of boronyl (BO) and the BO/H isolobal analogy build an interesting structural link between boron oxide clusters and hydrocarbons. Based upon global-minimum searches and first-principles electronic structural calculations, we present here the perfectly planar C 2v B5O5 + (1, 1A1), C 2v B5O5 (2, 2A1), and tetrahedral Cs B5O5 − (3, 1A′) clusters, which are the global minima of the systems. Structural and molecular orbital analyses indicate that C 2v B5O5 + (1) [B3O3(BO)2 +] and C 2v B5O5 (2) [B3O3(BO)2] feature an aromatic six-membered boroxol (B3O3) ring as the core with two equivalent boronyl terminals, similar to the recently reported boronyl boroxine D 3h B6O6 [B3O3(BO)3]; whereas Cs B5O5 − (3) [B(BO)3(OBO)−] is characterized with a tetrahedral B− center, terminated with three BO groups and one OBO unit, similar to the previously predicted boronyl methane Td B5O4 − [B(BO)4 −]. Alternatively, the 1–3 clusters can be viewed as the boron oxide analogs of phenyl cation C6H5 +, phenyl radical C6H5, and chloromethane CH3Cl, respectively. Chemical bonding analyses also reveal a dual three-center four-electron (3c-4e) π hyperbond in Cs B5O5 − (3). The infrared absorption spectra of B5O5 + (1), B5O5 (2), and B5O5 − (3) and anion photoelectron spectrum of B5O5 − (3) are predicted to facilitate their forthcoming experimental characterizations. The present work completes the B n O n +/0/− series for n = 1–6 and enriches the analogous relationship between boron oxides and hydrocarbons.
中文翻译:

关于B5O5 + / 0和B5O5-团簇的第一性原理研究:C6H5 + / 0和CH3Cl的氧化硼类似物
硼基(BO)的概念和BO / H异族类似物在氧化硼团簇和碳氢化合物之间建立了有趣的结构联系。基于全局最小的搜索和第一原理电子结构计算,我们在这里提出的完全平坦Ç 2 v 乙5 ø 5 +(1,1阿1),C ^ 2 v 乙5 ø 5(2,2甲1)和四面体ç小号 乙5 ø 5 - (3,1 A')集群是系统的全局最小值。结构和分子轨道分析表明,C 2 v B 5 O 5 +(1)[B 3 O 3(BO)2 + ]和C 2 v B 5 O 5(2)[B 3 O 3(BO)2 ]具有一个芳香族的六元环硼氧烷(B 3 O 3)环作为核心,带有两个当量的硼烷基末端,类似于最近报道的硼烷基环硼氧烷D 3 h B 6 O 6 [B 3 O 3(BO)3 ];而Ç小号 乙5 ø 5 - (3)[B(BO)3(OBO)- ]的特征在于与一个四面体乙-中心,用三个BO基团和一个OBO单元,类似于先前预测的硼羰甲烷终止Ť d 乙5 ö 4 - [B(BO)4 - ]。或者,1 – 3簇可以看作分别是苯基阳离子C 6 H 5 +,苯基自由基C 6 H 5和氯甲烷CH 3 Cl的氧化硼类似物。化学结合分析也揭示了在双三中心四电子(3C-4E)πhyperbond Ç小号 乙5 ø 5 - (3)。B的红外吸收光谱5 ø 5 +(1),B 5 ø 5(2),和B 5 ø 5 - (3B的)和阴离子光电子光谱5 ø 5 - (3)被预测为方便他们即将试验表征。目前的工作完成了n = 1-6的B n O n + / 0 /-级数,并丰富了氧化硼和碳氢化合物之间的相似关系。
更新日期:2015-08-13
中文翻译:

关于B5O5 + / 0和B5O5-团簇的第一性原理研究:C6H5 + / 0和CH3Cl的氧化硼类似物
硼基(BO)的概念和BO / H异族类似物在氧化硼团簇和碳氢化合物之间建立了有趣的结构联系。基于全局最小的搜索和第一原理电子结构计算,我们在这里提出的完全平坦Ç 2 v 乙5 ø 5 +(1,1阿1),C ^ 2 v 乙5 ø 5(2,2甲1)和四面体ç小号 乙5 ø 5 - (3,1 A')集群是系统的全局最小值。结构和分子轨道分析表明,C 2 v B 5 O 5 +(1)[B 3 O 3(BO)2 + ]和C 2 v B 5 O 5(2)[B 3 O 3(BO)2 ]具有一个芳香族的六元环硼氧烷(B 3 O 3)环作为核心,带有两个当量的硼烷基末端,类似于最近报道的硼烷基环硼氧烷D 3 h B 6 O 6 [B 3 O 3(BO)3 ];而Ç小号 乙5 ø 5 - (3)[B(BO)3(OBO)- ]的特征在于与一个四面体乙-中心,用三个BO基团和一个OBO单元,类似于先前预测的硼羰甲烷终止Ť d 乙5 ö 4 - [B(BO)4 - ]。或者,1 – 3簇可以看作分别是苯基阳离子C 6 H 5 +,苯基自由基C 6 H 5和氯甲烷CH 3 Cl的氧化硼类似物。化学结合分析也揭示了在双三中心四电子(3C-4E)πhyperbond Ç小号 乙5 ø 5 - (3)。B的红外吸收光谱5 ø 5 +(1),B 5 ø 5(2),和B 5 ø 5 - (3B的)和阴离子光电子光谱5 ø 5 - (3)被预测为方便他们即将试验表征。目前的工作完成了n = 1-6的B n O n + / 0 /-级数,并丰富了氧化硼和碳氢化合物之间的相似关系。





























 京公网安备 11010802027423号
京公网安备 11010802027423号