Synthesis ( IF 2.2 ) Pub Date : 2015-08-11 , DOI: 10.1055/s-0034-1381054 Yunxia Wang , Xiangdong Hu , Fenfen Xiao , Qing Zhang
|
Abstract
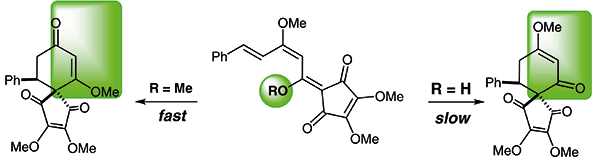
During synthetic studies toward bi-linderone, unexpected 6π-electrocyclizations of 4,5-dimethoxy-2-[(E)-1,3-dimethoxy-5-phenylpenta-2,4-dienylidene]cyclopent-4-ene-1,3-dione and 2-[(E)-1-hydroxy-3-methoxy-5-phenylpenta-2,4-dienylidene]-4,5-dimethoxycyclopent-4-ene-1,3-dione were observed. The cyclization of 4,5-dimethoxy-2-[(E)-1,3-dimethoxy-5-phenylpenta-2,4-dienylidene]cyclopent-4-ene-1,3-dione gave a rare example of a 6π-electrocyclization reaction that occurs readily at room temperature in the presence of silica gel. Meanwhile, the intramolecular hydrogen bonding existing in 2-[(E)-1-hydroxy-3-methoxy-5-phenylpenta-2,4-dienylidene]-4,5-dimethoxycyclopent-4-ene-1,3-dione afforded a strong contribution to the stability of the flat conformation, and this hampered the 6π-electrocyclization process. These 6π-electrocyclizations afforded a facile approach to highly congested spiro compounds.
During synthetic studies toward bi-linderone, unexpected 6π-electrocyclizations of 4,5-dimethoxy-2-[(E)-1,3-dimethoxy-5-phenylpenta-2,4-dienylidene]cyclopent-4-ene-1,3-dione and 2-[(E)-1-hydroxy-3-methoxy-5-phenylpenta-2,4-dienylidene]-4,5-dimethoxycyclopent-4-ene-1,3-dione were observed. The cyclization of 4,5-dimethoxy-2-[(E)-1,3-dimethoxy-5-phenylpenta-2,4-dienylidene]cyclopent-4-ene-1,3-dione gave a rare example of a 6π-electrocyclization reaction that occurs readily at room temperature in the presence of silica gel. Meanwhile, the intramolecular hydrogen bonding existing in 2-[(E)-1-hydroxy-3-methoxy-5-phenylpenta-2,4-dienylidene]-4,5-dimethoxycyclopent-4-ene-1,3-dione afforded a strong contribution to the stability of the flat conformation, and this hampered the 6π-electrocyclization process. These 6π-electrocyclizations afforded a facile approach to highly congested spiro compounds.
中文翻译:

对双林德龙的合成研究导致意外的6π电环化反应:高度拥堵的螺化合物的简便构建
摘要
在对双亚麻酮的合成研究过程中,4,5-二甲氧基-2-[((E)-1,3-二甲氧基-5-苯基戊-2,4-二烯叉]]环戊-4-烯-1发生了意外的6π电环化,观察到3-二酮和2-[((E)-1-羟基-3-甲氧基-5-苯基戊-2,4-二烯叉]]-4,5-二甲氧基环戊-4-烯-1,3-二酮。4,5-二甲氧基-2-[(E)-1,3-二甲氧基-5-苯基戊-2,4-二烯叉]环戊-4-烯-1,3-二酮的环化给出了一个罕见的6π例子-在硅胶存在下于室温下容易发生的电环化反应。同时,2-[(E)-1-羟基-3-甲氧基-5-苯基戊基-2,4-二烯叉] -4,5-二甲氧基环戊-4-烯-1,3-二酮对平面构象的稳定性有很强的贡献,阻碍了6π电环化过程。这些6π-电环化为高度拥挤的螺环化合物提供了一种简便的方法。
在对双亚麻酮的合成研究过程中,4,5-二甲氧基-2-[((E)-1,3-二甲氧基-5-苯基戊-2,4-二烯叉]]环戊-4-烯-1发生了意外的6π电环化,观察到3-二酮和2-[((E)-1-羟基-3-甲氧基-5-苯基戊-2,4-二烯叉]]-4,5-二甲氧基环戊-4-烯-1,3-二酮。4,5-二甲氧基-2-[(E)-1,3-二甲氧基-5-苯基戊-2,4-二烯叉]环戊-4-烯-1,3-二酮的环化给出了一个罕见的6π例子-在硅胶存在下于室温下容易发生的电环化反应。同时,2-[(E)-1-羟基-3-甲氧基-5-苯基戊基-2,4-二烯叉] -4,5-二甲氧基环戊-4-烯-1,3-二酮对平面构象的稳定性有很强的贡献,阻碍了6π电环化过程。这些6π-电环化为高度拥挤的螺环化合物提供了一种简便的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号