当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removing Cross-Linked Telopeptides Enhances the Production of Low-Molecular-Weight Collagen Peptides from Spent Hens
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1021/acs.jafc.7b02319 Hui Hong 1 , Shreyak Chaplot 1 , Meram Chalamaiah 1 , Bimol C. Roy 1 , Heather L. Bruce 1 , Jianping Wu 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2017-08-15 00:00:00 , DOI: 10.1021/acs.jafc.7b02319 Hui Hong 1 , Shreyak Chaplot 1 , Meram Chalamaiah 1 , Bimol C. Roy 1 , Heather L. Bruce 1 , Jianping Wu 1
Affiliation
|
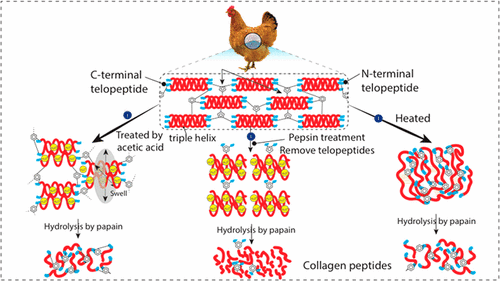
The low-molecular-weight (LMW) peptides derived from collagen have shown a potential for various nutritional and pharmaceutical applications. However, production of LMW peptides from vertebrate collagen remains a challenge. Herein, we report a new method to produce LMW collagen peptides using pepsin pretreatment that removed cross-linked telopeptides in collagen molecules. After the pretreatment, the proportion of LMW collagen peptides (<1.4 kDa) that were obtained from pepsin-soluble collagen increased to 32.59% compared to heat-soluble collagen peptides (16.10%). Fourier transform infrared spectroscopy results indicated that telopeptide cleavage retained the triple-helical conformation of collagen. Liquid chromatography–tandem mass spectrometry analysis suggested that Gly-X-Y (X is often proline, while Y is either hydroxyproline or hydroxylysine) repeats were not the main factors that hindered the enzymatic hydrolysis of collagen molecules. However, cross-link quantification demonstrated that trivalent cross-links that included pyridinolines and pyrroles were the primary obstacles to producing small peptides from collagen of spent hens. This study demonstrated for the first time that removing cross-linked telopeptides could enhance the production of LMW peptides from spent hen collagen, which is also of interest to manufacturers who produce LMW collagen peptides from other vertebrate animals, such as bovids and porcids.
中文翻译:

去除交联的端肽增强了从废母鸡生产低分子量胶原蛋白肽的能力
源自胶原的低分子量(LMW)肽已显示出在各种营养和药物应用中的潜力。然而,从脊椎动物胶原蛋白生产LMW肽仍然是一个挑战。在这里,我们报告了一种新的方法来使用胃蛋白酶预处理来生产LMW胶原蛋白肽,该酶去除了胶原分子中的交联的端肽。预处理后,从胃蛋白酶可溶的胶原蛋白获得的LMW胶原蛋白肽(<1.4 kDa)的比例与可溶的胶原蛋白肽(16.10%)相比增加到32.59%。傅里叶变换红外光谱结果表明,端肽裂解保留了胶原的三螺旋构象。液相色谱-串联质谱分析表明,Gly-XY(X通常为脯氨酸,虽然Y是羟脯氨酸或羟赖氨酸)重复不是阻碍胶原蛋白分子水解的主要因素。然而,交联定量证明包括吡啶啉和吡咯在内的三价交联是从废母鸡胶原蛋白生产小肽的主要障碍。这项研究首次证明,去除交联的端肽可以提高用过的母鸡胶原蛋白生产LMW肽的能力,这对于从其他脊椎动物(例如牛科动物和porcids)生产LMW胶原蛋白肽的制造商也很感兴趣。交联定量表明,包括吡啶啉和吡咯在内的三价交联是从废母鸡胶原蛋白生产小肽的主要障碍。这项研究首次证明,去除交联的端肽可以提高用过的母鸡胶原蛋白生产LMW肽的能力,这对于从其他脊椎动物(例如牛科动物和porcids)生产LMW胶原蛋白肽的制造商也很感兴趣。交联定量表明,包括吡啶啉和吡咯在内的三价交联是从废母鸡胶原蛋白生产小肽的主要障碍。这项研究首次证明,去除交联的端肽可以提高用过的母鸡胶原蛋白生产LMW肽的能力,这对于从其他脊椎动物(例如牛科动物和porcids)生产LMW胶原蛋白肽的制造商也很感兴趣。
更新日期:2017-08-15
中文翻译:

去除交联的端肽增强了从废母鸡生产低分子量胶原蛋白肽的能力
源自胶原的低分子量(LMW)肽已显示出在各种营养和药物应用中的潜力。然而,从脊椎动物胶原蛋白生产LMW肽仍然是一个挑战。在这里,我们报告了一种新的方法来使用胃蛋白酶预处理来生产LMW胶原蛋白肽,该酶去除了胶原分子中的交联的端肽。预处理后,从胃蛋白酶可溶的胶原蛋白获得的LMW胶原蛋白肽(<1.4 kDa)的比例与可溶的胶原蛋白肽(16.10%)相比增加到32.59%。傅里叶变换红外光谱结果表明,端肽裂解保留了胶原的三螺旋构象。液相色谱-串联质谱分析表明,Gly-XY(X通常为脯氨酸,虽然Y是羟脯氨酸或羟赖氨酸)重复不是阻碍胶原蛋白分子水解的主要因素。然而,交联定量证明包括吡啶啉和吡咯在内的三价交联是从废母鸡胶原蛋白生产小肽的主要障碍。这项研究首次证明,去除交联的端肽可以提高用过的母鸡胶原蛋白生产LMW肽的能力,这对于从其他脊椎动物(例如牛科动物和porcids)生产LMW胶原蛋白肽的制造商也很感兴趣。交联定量表明,包括吡啶啉和吡咯在内的三价交联是从废母鸡胶原蛋白生产小肽的主要障碍。这项研究首次证明,去除交联的端肽可以提高用过的母鸡胶原蛋白生产LMW肽的能力,这对于从其他脊椎动物(例如牛科动物和porcids)生产LMW胶原蛋白肽的制造商也很感兴趣。交联定量表明,包括吡啶啉和吡咯在内的三价交联是从废母鸡胶原蛋白生产小肽的主要障碍。这项研究首次证明,去除交联的端肽可以提高用过的母鸡胶原蛋白生产LMW肽的能力,这对于从其他脊椎动物(例如牛科动物和porcids)生产LMW胶原蛋白肽的制造商也很感兴趣。






























 京公网安备 11010802027423号
京公网安备 11010802027423号