当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissociative Photoionization of the Elusive Vinoxy Radical
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.jpca.7b04730
Jonathan D. Adams,Preston G. Scrape,Shih-Huang Lee,Laurie J. Butler
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.jpca.7b04730
Jonathan D. Adams,Preston G. Scrape,Shih-Huang Lee,Laurie J. Butler
|
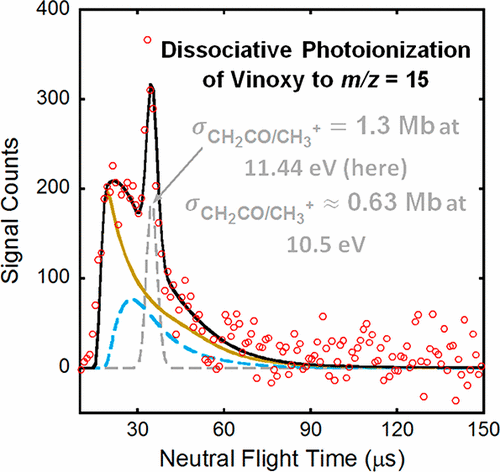
These experiments report the dissociative photoionization of vinoxy radicals to m/z = 15 and 29. In a crossed laser–molecular beam scattering apparatus, we induce C–Cl bond fission in 2-chloroacetaldehyde by photoexcitation at 157 nm. Our velocity measurements, combined with conservation of angular momentum, show that 21% of the C–Cl photofission events form vinoxy radicals that are stable to subsequent dissociation to CH3 + CO or H + ketene. Photoionization of these stable vinoxy radicals, identified by their velocities, which are momentum-matched with the higher-kinetic-energy Cl atom photofragments, shows that the vinoxy radicals dissociatively photoionize to give signal at m/z = 15 and 29. We calibrated the partial photoionization cross section of vinoxy to CH3+ relative to the bandwidth-averaged photoionization cross section of the Cl atom at 13.68 eV to put the partial photoionization cross sections on an absolute scale. The resulting bandwidth-averaged partial cross sections are 0.63 and 1.3 Mb at 10.5 and 11.44 eV, respectively. These values are consistent with the upper limit to the cross section estimated from a study by Savee et al. on the O(3P) + propene bimolecular reaction. We note that the uncertainty in these values is primarily dependent on the signal attributed to C–Cl primary photofission in the m/z = 35 (Cl+) time-of-flight data. While the value is a rough estimate, the bandwidth-averaged partial photoionization cross section of vinoxy to HCO+ calculated from the signal at m/z = 29 at 11.53 eV is approximately half that of vinoxy to CH3+. We also present critical points on the potential energy surface of the vinoxy cation calculated at the G4//B3LYP/6-311++G(3df,2p) level of theory to support the observation of dissociative ionization of vinoxy to both CH3+ and HCO+.
中文翻译:

难以捉摸的乙烯基自由基的解离光电离
这些实验报告了vinoxy自由基的解离光电离,分别为m / z = 15和29。在交叉的激光-分子束散射仪中,我们通过在157 nm处进行光激发在C-Cl键裂变中诱导2-氯乙醛的裂变。我们的速度测量结果与角动量守恒相结合,表明21%的C–Cl光裂变事件形成了对随后解离为CH 3 + CO或H +乙烯酮而言稳定的乙烯基氧基。这些稳定的vinoxy自由基的光电离,由它们的速度确定,其动量与较高运动能的Cl原子光碎片相匹配,表明vinoxy自由基离解性光电离以在m / z处给出信号分别等于15和29。我们相对于Cl原子的带宽平均光电离横截面在13.68 eV处,将vinoxy的部分光电离横截面校准为CH 3 +,以使局部光电离横截面处于绝对比例。所得的平均带宽局部截面在10.5和11.44 eV时分别为0.63和1.3 Mb。这些值与Savee等人的研究估计的横截面上限一致。O(3 P)+丙烯双分子反应。我们注意到,这些值的不确定性主要取决于m / z = 35(Cl +)飞行时间数据。尽管该值是一个粗略的估计值,但根据m / z = 29处的信号在11.53 eV处计算得出的vinoxy到HCO +的带宽平均部分光电离截面大约是vinoxy到CH 3 +的一半。我们还介绍了在G4 // B3LYP / 6-311 ++ G(3df,2p)理论水平上计算得出的vinoxy阳离子势能面上的临界点,以支持对vinoxy分解为CH 3 +的离解电离的观察。和HCO +。
更新日期:2017-08-14
中文翻译:

难以捉摸的乙烯基自由基的解离光电离
这些实验报告了vinoxy自由基的解离光电离,分别为m / z = 15和29。在交叉的激光-分子束散射仪中,我们通过在157 nm处进行光激发在C-Cl键裂变中诱导2-氯乙醛的裂变。我们的速度测量结果与角动量守恒相结合,表明21%的C–Cl光裂变事件形成了对随后解离为CH 3 + CO或H +乙烯酮而言稳定的乙烯基氧基。这些稳定的vinoxy自由基的光电离,由它们的速度确定,其动量与较高运动能的Cl原子光碎片相匹配,表明vinoxy自由基离解性光电离以在m / z处给出信号分别等于15和29。我们相对于Cl原子的带宽平均光电离横截面在13.68 eV处,将vinoxy的部分光电离横截面校准为CH 3 +,以使局部光电离横截面处于绝对比例。所得的平均带宽局部截面在10.5和11.44 eV时分别为0.63和1.3 Mb。这些值与Savee等人的研究估计的横截面上限一致。O(3 P)+丙烯双分子反应。我们注意到,这些值的不确定性主要取决于m / z = 35(Cl +)飞行时间数据。尽管该值是一个粗略的估计值,但根据m / z = 29处的信号在11.53 eV处计算得出的vinoxy到HCO +的带宽平均部分光电离截面大约是vinoxy到CH 3 +的一半。我们还介绍了在G4 // B3LYP / 6-311 ++ G(3df,2p)理论水平上计算得出的vinoxy阳离子势能面上的临界点,以支持对vinoxy分解为CH 3 +的离解电离的观察。和HCO +。

































 京公网安备 11010802027423号
京公网安备 11010802027423号