当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Porous Al Current Collector for Dendrite-Free Na Metal Anodes
Nano Letters ( IF 9.6 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.nanolett.7b03185 Shan Liu 1 , Shan Tang 2 , Xinyue Zhang 1 , Aoxuan Wang 1 , Quan-Hong Yang 1 , Jiayan Luo 1
Nano Letters ( IF 9.6 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.nanolett.7b03185 Shan Liu 1 , Shan Tang 2 , Xinyue Zhang 1 , Aoxuan Wang 1 , Quan-Hong Yang 1 , Jiayan Luo 1
Affiliation
|
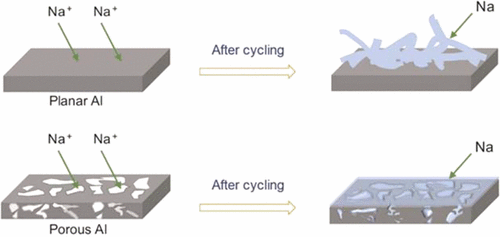
Na-based batteries are proposed as promising energy storage candidates for beyond Li-ion technology due to the higher natural earth of Na metal. For its high capacity and low potential, Na metal may carve itself a niche when directly used as anodes. Similar to or even more problematic than Li, however, uneven plating/stripping of Na leads to dendrite formation. As the plating substrates, current collectors have a paramount influence on the Na plating/stripping behaviors. Here we propose porous Al current collectors as the plating substrate to suppress Na dendrites. Al does not alloy with Na. It is advantageous over Cu current collectors in terms of cost and weight. The interconnected porous structure can increase available surface for Na to nucleate and decrease the Na+ flux distribution, leading to homogeneous plating. The Na metal anodes can run for over 1000 cycles on porous Al with a low and stable voltage hysteresis and their average plating/stripping Coulombic efficiency was above 99.9%, which is greatly improved compared to planar Al. We used the porous Al for Na–O2, Na–Na3V2(PO4)3 cells with low Na amount and anode free Na–TiS2 batteries and anticipate that using this strategy can be combined with further electrolyte and cathodes to develop high performance Na-based batteries.
中文翻译:

无树突钠金属阳极的多孔铝集电器
由于钠金属的天然含量较高,因此建议将钠基电池作为超越锂离子技术的有前途的储能材料。由于其高容量和低电势,金属钠在直接用作阳极时可能会开拓自己的市场。与Li相似,甚至比Li更成问题,Na的不均匀电镀/剥离会导致枝晶形成。作为电镀基板,集电器对Na电镀/剥离行为具有至关重要的影响。在这里,我们提出了多孔铝集电器作为电镀基体,以抑制钠树枝状晶体。Al不与Na形成合金。就成本和重量而言,它比铜集电器更具优势。相互连接的多孔结构可以增加可用的Na形核表面,并减少Na +助焊剂分布,导致镀层均匀。Na金属阳极可以在具有低且稳定的电压滞后的多孔Al上运行超过1000个循环,并且它们的平均电镀/剥离库仑效率高于99.9%,与平面Al相比有很大提高。我们将多孔Al用于Na含量低的Na–O 2,Na–Na 3 V 2(PO 4)3电池和无阳极的Na–TiS 2电池,并预计使用该策略可以与其他电解质和阴极结合使用开发高性能钠基电池。
更新日期:2017-08-15
中文翻译:

无树突钠金属阳极的多孔铝集电器
由于钠金属的天然含量较高,因此建议将钠基电池作为超越锂离子技术的有前途的储能材料。由于其高容量和低电势,金属钠在直接用作阳极时可能会开拓自己的市场。与Li相似,甚至比Li更成问题,Na的不均匀电镀/剥离会导致枝晶形成。作为电镀基板,集电器对Na电镀/剥离行为具有至关重要的影响。在这里,我们提出了多孔铝集电器作为电镀基体,以抑制钠树枝状晶体。Al不与Na形成合金。就成本和重量而言,它比铜集电器更具优势。相互连接的多孔结构可以增加可用的Na形核表面,并减少Na +助焊剂分布,导致镀层均匀。Na金属阳极可以在具有低且稳定的电压滞后的多孔Al上运行超过1000个循环,并且它们的平均电镀/剥离库仑效率高于99.9%,与平面Al相比有很大提高。我们将多孔Al用于Na含量低的Na–O 2,Na–Na 3 V 2(PO 4)3电池和无阳极的Na–TiS 2电池,并预计使用该策略可以与其他电解质和阴极结合使用开发高性能钠基电池。































 京公网安备 11010802027423号
京公网安备 11010802027423号