当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chirality Transfer from Chiral Monoamines to an m-Phthalic Diamide-Linked Zinc Bisporphyrinate with a Benzylamide Substituent
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.inorgchem.7b00815 Qingyun Hu 1 , Congcong Zhuo 1 , Yong Wang 1 , Chuanjiang Hu 1, 2 , Jianping Lang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acs.inorgchem.7b00815 Qingyun Hu 1 , Congcong Zhuo 1 , Yong Wang 1 , Chuanjiang Hu 1, 2 , Jianping Lang 1
Affiliation
|
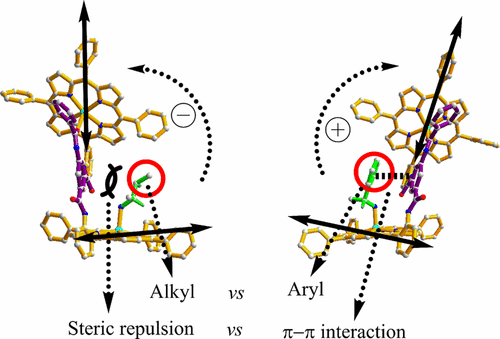
An m-phthalic diamide-linked bisporphyrin with a benzylamide substituent has been designed and synthesized. It has two types of carbonyl groups. In the solution of this zinc bisporphyrinate, these carbonyl groups are involved in the formation of two different Zn–O coordination interactions: one is formed between neighboring zinc bisporphyrinates; another is formed within zinc bisporphyrinate. The chirality sensing abilities of this zinc porphyrinate to a number of chiral monoamines have been examined. When zinc bisporphyrinate was mixed with a series of chiral monoamines, the signs of the circular dichroism spectra for the chiral monoamines of the same handedness with an aryl group as the substituent are just opposite to those with an alkyl group as the substituent. NMR studies reveal that stepwise coordinations lead to 1:1 and 1:2 host–guest complexes. The structure of the 1:1 host–guest complex was confirmed by crystallography, it is the first time that a 1:1 host–guest complex formed between zinc bisporphyrinate and a chiral monoamine has been crystallographically characterized. The structure reveals that there is an intramolecular hydrogen bond between the amide oxygen and the coordinated NH2. We further investigated the chirality transfer mechanism by density functional theory calculations. Our studies suggest that the interactions between the linker and guests in this bisporphyrin system are crucial in the chirality transfer process, and the nature of the bulkiest substituent of chiral monoamines makes a difference. For R-type guests, with an alkyl group, the steric repulsion makes the conformer A more energetically favorable, which leads to the anticlockwise twist and negative Cotton effect. However, with an aryl group, the π–π interaction makes the conformer B more energetically favorable, which leads to the clockwise twist and positive Cotton effect.
中文翻译:

自Chiral单胺手性转移到米-邻二酰胺联锌Bisporphyrinate用苄基酰胺取代基
安m已经设计并合成了具有苄基酰胺取代基的邻苯二甲酸二酰胺连接的双卟啉。它具有两种类型的羰基。在这种双卟啉锌溶液中,这些羰基参与了两种不同的Zn-O配位相互作用:一种是在相邻的双卟啉锌之间形成;另一种是在相邻的双卟啉锌之间形成。另一个在双卟啉锌中形成。已经检查了该卟啉锌对许多手性单胺的手性感测能力。当双卟啉锌与一系列手性单胺混合时,具有芳基作为取代基的相同手性的手性单胺的圆二色性谱的符号与烷基作为取代基的手性单胺的圆二色性谱正好相反。NMR研究表明,逐步配位导致1:1和1:2主客复合体。晶体学证实了1:1主体-客体复合物的结构,这是首次对双卟啉锌和手性单胺之间形成的1:1主体-客体复合物进行了晶体学表征。结构表明在酰胺氧和配位的NH之间存在分子内氢键2。我们通过密度泛函理论计算进一步研究了手性传递机理。我们的研究表明,在该双卟啉体系中,连接基与客体之间的相互作用在手性转移过程中至关重要,而手性单胺中最大的取代基的性质也有所不同。对于带有烷基的R型客体,空间排斥使构象异构体A在能量上更有利,从而导致逆时针扭曲和负面的Cotton效应。但是,使用芳基时,π-π相互作用使构象异构体B在能量上更有利,从而导致顺时针扭曲和正棉花效应。
更新日期:2017-08-14
中文翻译:

自Chiral单胺手性转移到米-邻二酰胺联锌Bisporphyrinate用苄基酰胺取代基
安m已经设计并合成了具有苄基酰胺取代基的邻苯二甲酸二酰胺连接的双卟啉。它具有两种类型的羰基。在这种双卟啉锌溶液中,这些羰基参与了两种不同的Zn-O配位相互作用:一种是在相邻的双卟啉锌之间形成;另一种是在相邻的双卟啉锌之间形成。另一个在双卟啉锌中形成。已经检查了该卟啉锌对许多手性单胺的手性感测能力。当双卟啉锌与一系列手性单胺混合时,具有芳基作为取代基的相同手性的手性单胺的圆二色性谱的符号与烷基作为取代基的手性单胺的圆二色性谱正好相反。NMR研究表明,逐步配位导致1:1和1:2主客复合体。晶体学证实了1:1主体-客体复合物的结构,这是首次对双卟啉锌和手性单胺之间形成的1:1主体-客体复合物进行了晶体学表征。结构表明在酰胺氧和配位的NH之间存在分子内氢键2。我们通过密度泛函理论计算进一步研究了手性传递机理。我们的研究表明,在该双卟啉体系中,连接基与客体之间的相互作用在手性转移过程中至关重要,而手性单胺中最大的取代基的性质也有所不同。对于带有烷基的R型客体,空间排斥使构象异构体A在能量上更有利,从而导致逆时针扭曲和负面的Cotton效应。但是,使用芳基时,π-π相互作用使构象异构体B在能量上更有利,从而导致顺时针扭曲和正棉花效应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号