当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ATR inhibition facilitates targeting of leukemia dependence on convergent nucleotide biosynthetic pathways.
Nature Communications ( IF 14.7 ) Pub Date : 2017-08-14 , DOI: 10.1038/s41467-017-00221-3 Thuc M. Le , Soumya Poddar , Joseph R. Capri , Evan R. Abt , Woosuk Kim , Liu Wei , Nhu T. Uong , Chloe M. Cheng , Daniel Braas , Mina Nikanjam , Peter Rix , Daria Merkurjev , Jesse Zaretsky , Harley I. Kornblum , Antoni Ribas , Harvey R. Herschman , Julian Whitelegge , Kym F. Faull , Timothy R. Donahue , Johannes Czernin , Caius G. Radu
Nature Communications ( IF 14.7 ) Pub Date : 2017-08-14 , DOI: 10.1038/s41467-017-00221-3 Thuc M. Le , Soumya Poddar , Joseph R. Capri , Evan R. Abt , Woosuk Kim , Liu Wei , Nhu T. Uong , Chloe M. Cheng , Daniel Braas , Mina Nikanjam , Peter Rix , Daria Merkurjev , Jesse Zaretsky , Harley I. Kornblum , Antoni Ribas , Harvey R. Herschman , Julian Whitelegge , Kym F. Faull , Timothy R. Donahue , Johannes Czernin , Caius G. Radu
|
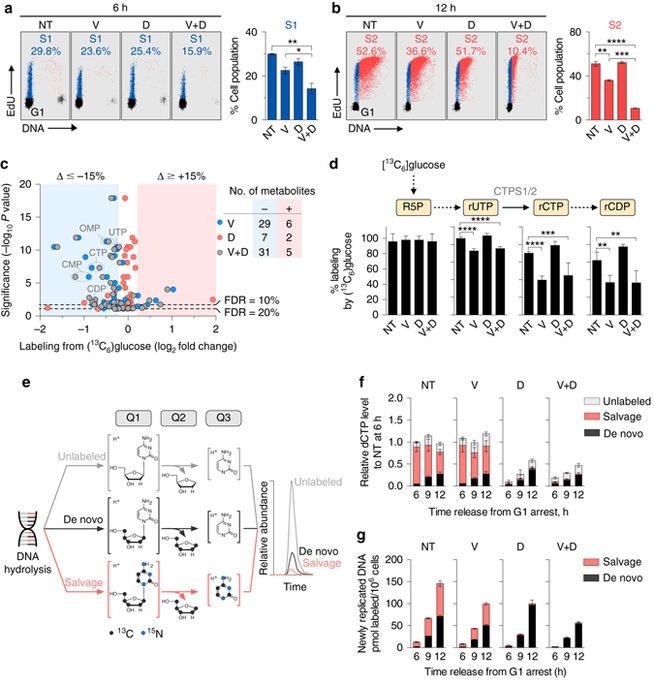
Leukemia cells rely on two nucleotide biosynthetic pathways, de novo and salvage, to produce dNTPs for DNA replication. Here, using metabolomic, proteomic, and phosphoproteomic approaches, we show that inhibition of the replication stress sensing kinase ataxia telangiectasia and Rad3-related protein (ATR) reduces the output of both de novo and salvage pathways by regulating the activity of their respective rate-limiting enzymes, ribonucleotide reductase (RNR) and deoxycytidine kinase (dCK), via distinct molecular mechanisms. Quantification of nucleotide biosynthesis in ATR-inhibited acute lymphoblastic leukemia (ALL) cells reveals substantial remaining de novo and salvage activities, and could not eliminate the disease in vivo. However, targeting these remaining activities with RNR and dCK inhibitors triggers lethal replication stress in vitro and long-term disease-free survival in mice with B-ALL, without detectable toxicity. Thus the functional interplay between alternative nucleotide biosynthetic routes and ATR provides therapeutic opportunities in leukemia and potentially other cancers.Leukemic cells depend on the nucleotide synthesis pathway to proliferate. Here the authors use metabolomics and proteomics to show that inhibition of ATR reduced the activity of these pathways thus providing a valuable therapeutic target in leukemia.
中文翻译:

ATR抑制促进白血病对收敛核苷酸生物合成途径的依赖。
白血病细胞依靠从头和挽救这两种核苷酸生物合成途径来产生用于DNA复制的dNTP。在这里,我们使用代谢组学,蛋白质组学和磷酸化蛋白质组学方法,表明抑制复制应激感应激酶共济失调毛细血管扩张和Rad3相关蛋白(ATR)可以通过调节各自的速率活性来降低从头途径和挽救途径的输出,限制酶,核糖核苷酸还原酶(RNR)和脱氧胞苷激酶(dCK),通过独特的分子机制。定量ATR抑制的急性淋巴细胞白血病(ALL)细胞中核苷酸生物合成的定量显示大量剩余的从头和挽救活动,并不能消除体内疾病。然而,使用RNR和dCK抑制剂靶向这些剩余的活性,可在B-ALL小鼠体内触发致命的复制应激和长期无病生存,且无可检测的毒性。因此,替代核苷酸生物合成途径与ATR之间的功能相互作用为白血病和潜在的其他癌症提供了治疗机会。白血病细胞依赖于核苷酸合成途径进行增殖。在这里,作者使用代谢组学和蛋白质组学来证明对ATR的抑制会降低这些途径的活性,从而为白血病提供有价值的治疗靶标。白血病细胞增殖依赖于核苷酸合成途径。在这里,作者使用代谢组学和蛋白质组学来证明对ATR的抑制会降低这些途径的活性,从而为白血病提供有价值的治疗靶标。白血病细胞依赖于核苷酸合成途径进行增殖。在这里,作者使用代谢组学和蛋白质组学来证明对ATR的抑制会降低这些途径的活性,从而为白血病提供有价值的治疗靶标。
更新日期:2017-08-14
中文翻译:

ATR抑制促进白血病对收敛核苷酸生物合成途径的依赖。
白血病细胞依靠从头和挽救这两种核苷酸生物合成途径来产生用于DNA复制的dNTP。在这里,我们使用代谢组学,蛋白质组学和磷酸化蛋白质组学方法,表明抑制复制应激感应激酶共济失调毛细血管扩张和Rad3相关蛋白(ATR)可以通过调节各自的速率活性来降低从头途径和挽救途径的输出,限制酶,核糖核苷酸还原酶(RNR)和脱氧胞苷激酶(dCK),通过独特的分子机制。定量ATR抑制的急性淋巴细胞白血病(ALL)细胞中核苷酸生物合成的定量显示大量剩余的从头和挽救活动,并不能消除体内疾病。然而,使用RNR和dCK抑制剂靶向这些剩余的活性,可在B-ALL小鼠体内触发致命的复制应激和长期无病生存,且无可检测的毒性。因此,替代核苷酸生物合成途径与ATR之间的功能相互作用为白血病和潜在的其他癌症提供了治疗机会。白血病细胞依赖于核苷酸合成途径进行增殖。在这里,作者使用代谢组学和蛋白质组学来证明对ATR的抑制会降低这些途径的活性,从而为白血病提供有价值的治疗靶标。白血病细胞增殖依赖于核苷酸合成途径。在这里,作者使用代谢组学和蛋白质组学来证明对ATR的抑制会降低这些途径的活性,从而为白血病提供有价值的治疗靶标。白血病细胞依赖于核苷酸合成途径进行增殖。在这里,作者使用代谢组学和蛋白质组学来证明对ATR的抑制会降低这些途径的活性,从而为白血病提供有价值的治疗靶标。






























 京公网安备 11010802027423号
京公网安备 11010802027423号