当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inefficient Ribosomal Skipping Enables Simultaneous Secretion and Display of Proteins in Saccharomyces cerevisiae
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acssynbio.7b00144 Carlos A Cruz-Teran 1 , Karthik Tiruthani 1 , Adam Mischler 1 , Balaji M Rao 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2017-08-14 00:00:00 , DOI: 10.1021/acssynbio.7b00144 Carlos A Cruz-Teran 1 , Karthik Tiruthani 1 , Adam Mischler 1 , Balaji M Rao 1
Affiliation
|
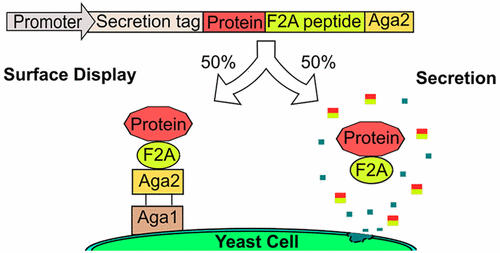
The need for recombinant expression of soluble protein slows the validation of engineered proteins isolated from combinatorial libraries and limits the number of protein variants evaluated. To overcome this bottleneck, we describe a system for simultaneous cell surface display and soluble secretion of proteins in Saccharomyces cerevisiae based on inefficient ribosomal skipping. Ribosomal skipping mediated by “self-cleaving” 2A peptides produces two proteins from a single open reading frame. Incorporation of the F2A peptide sequence—with ∼50% efficiency of ribosomal skipping—between the protein of interest and the yeast cell wall protein Aga2 results in simultaneous expression of both the solubly secreted protein and the protein–Aga2 fusion that is tethered to the yeast cell surface. We show that binding proteins derived from the Sso7d scaffold and the homodimeric enzyme glucose oxidase can be simultaneously secreted solubly and expressed as yeast cell surface fusions using the F2A-based system. Furthermore, a combinatorial library of Sso7d mutants can be screened to isolate binders with higher affinity for a model target (lysozyme), and the pool of higher affinity binders can be characterized in soluble form. Significantly, we show that both N- and C-terminal fusions to Aga2 can be simultaneously secreted solubly and displayed on the cell surface; this is particularly advantageous because protein functionality can be affected by the specific position of Aga2 in the protein fusion. We expect that the F2A-based yeast surface display and secretion system will be a useful tool for protein engineering and enable efficient characterization of individual clones isolated from combinatorial libraries.
中文翻译:

低效的核糖体跳跃使得酿酒酵母能够同时分泌和展示蛋白质
可溶性蛋白重组表达的需要减缓了从组合文库中分离的工程蛋白的验证,并限制了评估的蛋白变体的数量。为了克服这一瓶颈,我们描述了一种基于低效核糖体跳跃的酿酒酵母中同时细胞表面展示和蛋白质可溶性分泌的系统。由“自切割”2A 肽介导的核糖体跳跃从单个开放阅读框产生两种蛋白质。在目标蛋白和酵母细胞壁蛋白 Aga2 之间掺入 F2A 肽序列(核糖体跳跃效率约为 50%),导致可溶性分泌蛋白和与酵母相连的蛋白 Aga2 融合物同时表达细胞表面。我们证明,使用基于 F2A 的系统,源自 Sso7d 支架和同型二聚体酶葡萄糖氧化酶的结合蛋白可以同时溶解分泌并表达为酵母细胞表面融合物。此外,可以筛选Sso7d突变体的组合文库以分离对模型靶标(溶菌酶)具有更高亲和力的结合物,并且可以以可溶形式表征更高亲和力结合物库。值得注意的是,我们发现 Aga2 的 N 端和 C 端融合体可以同时溶解分泌并展示在细胞表面;这是特别有利的,因为蛋白质功能性会受到蛋白质融合体中 Aga2 的特定位置的影响。我们期望基于 F2A 的酵母表面展示和分泌系统将成为蛋白质工程的有用工具,并能够有效地表征从组合文库中分离的单个克隆。
更新日期:2017-08-14
中文翻译:

低效的核糖体跳跃使得酿酒酵母能够同时分泌和展示蛋白质
可溶性蛋白重组表达的需要减缓了从组合文库中分离的工程蛋白的验证,并限制了评估的蛋白变体的数量。为了克服这一瓶颈,我们描述了一种基于低效核糖体跳跃的酿酒酵母中同时细胞表面展示和蛋白质可溶性分泌的系统。由“自切割”2A 肽介导的核糖体跳跃从单个开放阅读框产生两种蛋白质。在目标蛋白和酵母细胞壁蛋白 Aga2 之间掺入 F2A 肽序列(核糖体跳跃效率约为 50%),导致可溶性分泌蛋白和与酵母相连的蛋白 Aga2 融合物同时表达细胞表面。我们证明,使用基于 F2A 的系统,源自 Sso7d 支架和同型二聚体酶葡萄糖氧化酶的结合蛋白可以同时溶解分泌并表达为酵母细胞表面融合物。此外,可以筛选Sso7d突变体的组合文库以分离对模型靶标(溶菌酶)具有更高亲和力的结合物,并且可以以可溶形式表征更高亲和力结合物库。值得注意的是,我们发现 Aga2 的 N 端和 C 端融合体可以同时溶解分泌并展示在细胞表面;这是特别有利的,因为蛋白质功能性会受到蛋白质融合体中 Aga2 的特定位置的影响。我们期望基于 F2A 的酵母表面展示和分泌系统将成为蛋白质工程的有用工具,并能够有效地表征从组合文库中分离的单个克隆。































 京公网安备 11010802027423号
京公网安备 11010802027423号