Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of the Human Phosphatase PTPN4 by the inter-domain linker connecting the PDZ and the phosphatase domains.
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Aug-11 , DOI: 10.1038/s41598-017-08193-6
Célia Caillet-Saguy , Angelo Toto , Raphael Guerois , Pierre Maisonneuve , Eva di Silvio , Kristi Sawyer , Stefano Gianni , Nicolas Wolff
Scientific Reports ( IF 3.8 ) Pub Date : 2017-Aug-11 , DOI: 10.1038/s41598-017-08193-6
Célia Caillet-Saguy , Angelo Toto , Raphael Guerois , Pierre Maisonneuve , Eva di Silvio , Kristi Sawyer , Stefano Gianni , Nicolas Wolff
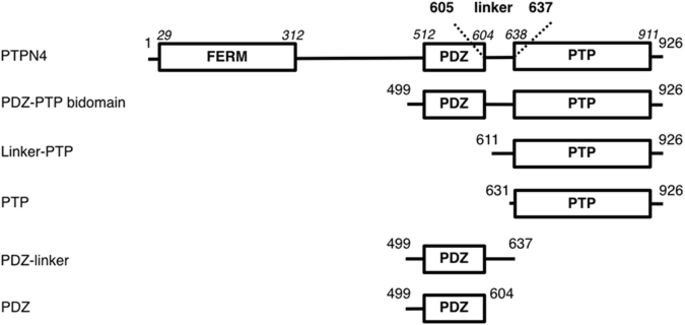
|
Human protein tyrosine phosphatase non-receptor type 4 (PTPN4) has been shown to prevent cell death. The active form of human PTPN4 consists of two globular domains, a PDZ (PSD-95/Dlg/ZO-1) domain and a phosphatase domain, tethered by a flexible linker. Targeting its PDZ domain abrogates this protection and triggers apoptosis. We previously demonstrated that the PDZ domain inhibits the phosphatase activity of PTPN4 and that the mere binding of a PDZ ligand is sufficient to release the catalytic inhibition. We demonstrate here that the linker connecting the PDZ domain and the phosphatase domain is involved in the regulation of the phosphatase activity in both PDZ-related inhibition and PDZ ligand-related activation events. We combined bioinformatics and kinetic studies to decipher the role of the linker in the PTPN4 activity. By comparing orthologous sequences, we identified a conserved patch of hydrophobic residues in the linker. We showed that mutations in this patch affect the regulation of the PTPN4 bidomain indicating that the PDZ-PDZ ligand regulation of PTPN4 is a linker-mediated mechanism. However, the mutations do not alter the binding of the PDZ ligand. This study strengthens the notion that inter-domain linker can be of functional importance in enzyme regulation of large multi-domain proteins.
中文翻译:

通过连接PDZ和磷酸酶结构域的域间接头调节人磷酸酶PTPN4。
人类蛋白酪氨酸磷酸酶非受体4型(PTPN4)已被证明可以防止细胞死亡。人PTPN4的活性形式由两个球形结构域组成,一个PDZ(PSD-95 / Dlg / ZO-1)结构域和一个磷酸酶结构域,由柔性连接子束缚。靶向其PDZ结构域取消了这种保护并触发了细胞凋亡。我们先前证明,PDZ结构域抑制PTPN4的磷酸酶活性,而PDZ配体的仅仅结合就足以释放催化抑制作用。我们在这里证明,连接PDZ结构域和磷酸酶结构域的接头参与PDZ相关的抑制和PDZ配体相关的激活事件中的磷酸酶活性的调节。我们结合了生物信息学和动力学研究来破译接头在PTPN4活性中的作用。通过比较直系同源序列,我们确定了接头中疏水残基的保守补丁。我们显示该补丁中的突变会影响PTPN4双结构域的调控,表明PTPN4的PDZ-PDZ配体调控是一种连接子介导的机制。但是,突变不会改变PDZ配体的结合。这项研究强化了域间连接子在大型多域蛋白的酶调控中可能具有功能重要性的观念。
更新日期:2017-08-11
中文翻译:

通过连接PDZ和磷酸酶结构域的域间接头调节人磷酸酶PTPN4。
人类蛋白酪氨酸磷酸酶非受体4型(PTPN4)已被证明可以防止细胞死亡。人PTPN4的活性形式由两个球形结构域组成,一个PDZ(PSD-95 / Dlg / ZO-1)结构域和一个磷酸酶结构域,由柔性连接子束缚。靶向其PDZ结构域取消了这种保护并触发了细胞凋亡。我们先前证明,PDZ结构域抑制PTPN4的磷酸酶活性,而PDZ配体的仅仅结合就足以释放催化抑制作用。我们在这里证明,连接PDZ结构域和磷酸酶结构域的接头参与PDZ相关的抑制和PDZ配体相关的激活事件中的磷酸酶活性的调节。我们结合了生物信息学和动力学研究来破译接头在PTPN4活性中的作用。通过比较直系同源序列,我们确定了接头中疏水残基的保守补丁。我们显示该补丁中的突变会影响PTPN4双结构域的调控,表明PTPN4的PDZ-PDZ配体调控是一种连接子介导的机制。但是,突变不会改变PDZ配体的结合。这项研究强化了域间连接子在大型多域蛋白的酶调控中可能具有功能重要性的观念。

































 京公网安备 11010802027423号
京公网安备 11010802027423号