当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxazine Ring-Related Vibrational Modes of Benzoxazine Monomers Using Fully Aromatically Substituted, Deuterated, 15N Isotope Exchanged, and Oxazine-Ring-Substituted Compounds and Theoretical Calculations
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1021/acs.jpca.7b05249 Lu Han 1 , Daniela Iguchi 1 , Phwey Gil 2 , Tyler R. Heyl 1 , Victoria M. Sedwick 1 , Carlos R. Arza 1 , Seishi Ohashi 1 , Daniel J. Lacks 2 , Hatsuo Ishida 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1021/acs.jpca.7b05249 Lu Han 1 , Daniela Iguchi 1 , Phwey Gil 2 , Tyler R. Heyl 1 , Victoria M. Sedwick 1 , Carlos R. Arza 1 , Seishi Ohashi 1 , Daniel J. Lacks 2 , Hatsuo Ishida 1
Affiliation
|
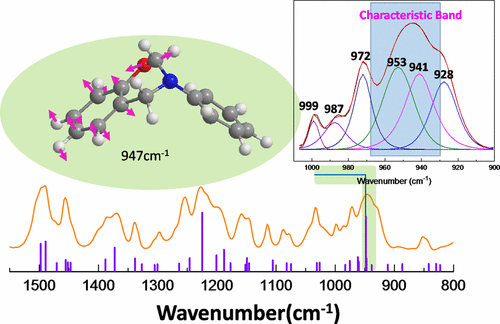
Polymerization of benzoxazine resins is indicated by the disappearance of a 960–900 cm–1 band in infrared spectroscopy (IR). Historically, this band was assigned to the C–H out-of-plane bending of the benzene to which the oxazine ring is attached. This study shows that this band is a mixture of the O–C2 stretching of the oxazine ring and the phenolic ring vibrational modes. Vibrational frequencies of 3-phenyl-3,4-dihydro-2H-benzo[e][1,3]oxazine (PH-a) and 3-(tert-butyl)-3,4-dihydro-2H-benzo[e][1,3]oxazine (PH-t) are compared with isotope-exchanged and all-substituted compounds. Deuterated benzoxazine monomers, 15N-isotope exchanged benzoxazine monomers, and all-substituted benzoxazine monomers without aromatic C–H groups are synthesized and studied meticulously. The various isotopic-exchanges involved deuteration around the benzene ring of phenol, selective deuteration of each CH2 in the O–CH2–N (2) and N–CH2–Ar (4) positions on the oxazine ring, or simultaneous deuteration of both positions. The chemical structures were confirmed by 1H nuclear magnetic resonance spectroscopy (1H NMR). The IR and Raman spectra of each compound are compared. Further analysis of 15N isotope-exchanged PH-a indicates the influence of the nitrogen isotope on the band position, both experimentally and theoretically. This finding is important for polymerization studies of benzoxazines that utilize vibrational spectroscopy.
中文翻译:

使用完全芳香取代,氘代,15 N同位素交换和恶嗪环取代的化合物与恶嗪环相关的恶嗪环相关振动模式和理论计算
红外光谱(IR)中960–900 cm –1谱带的消失表明了苯并恶嗪树脂的聚合。从历史上看,该谱带归属于与恶嗪环相连的苯的CH平面弯曲。这项研究表明,该带是恶嗪环的O–C 2拉伸和酚环振动模式的混合。3-苯基-3,4-二氢-2 H-苯并[ e ] [1,3]恶嗪(PH-a)和3-(叔丁基)-3,4-二氢-2 H-苯并的振动频率[ e ] [1,3]恶嗪(PH-t)与同位素交换和全取代的化合物进行比较。合成并进行了精细的研究,包括氘代苯并恶嗪单体,15种N-同位素交换的苯并恶嗪单体和全取代的不具有芳族C–H基团的苯并恶嗪单体。各种同位素交换包括苯酚的苯环周围的氘化,恶嗪环上O– CH 2 –N(2)和N– CH 2 –Ar(4)位置的每个CH 2的选择性氘化或同时氘化两个职位。化学结构通过1 H核磁共振波谱(1 H NMR)确认。比较每种化合物的IR和拉曼光谱。进一步分析在实验和理论上,15 N同位素交换的PH-a表示氮同位素对能带位置的影响。该发现对于利用振动光谱的苯并恶嗪的聚合研究很重要。
更新日期:2017-08-11
中文翻译:

使用完全芳香取代,氘代,15 N同位素交换和恶嗪环取代的化合物与恶嗪环相关的恶嗪环相关振动模式和理论计算
红外光谱(IR)中960–900 cm –1谱带的消失表明了苯并恶嗪树脂的聚合。从历史上看,该谱带归属于与恶嗪环相连的苯的CH平面弯曲。这项研究表明,该带是恶嗪环的O–C 2拉伸和酚环振动模式的混合。3-苯基-3,4-二氢-2 H-苯并[ e ] [1,3]恶嗪(PH-a)和3-(叔丁基)-3,4-二氢-2 H-苯并的振动频率[ e ] [1,3]恶嗪(PH-t)与同位素交换和全取代的化合物进行比较。合成并进行了精细的研究,包括氘代苯并恶嗪单体,15种N-同位素交换的苯并恶嗪单体和全取代的不具有芳族C–H基团的苯并恶嗪单体。各种同位素交换包括苯酚的苯环周围的氘化,恶嗪环上O– CH 2 –N(2)和N– CH 2 –Ar(4)位置的每个CH 2的选择性氘化或同时氘化两个职位。化学结构通过1 H核磁共振波谱(1 H NMR)确认。比较每种化合物的IR和拉曼光谱。进一步分析在实验和理论上,15 N同位素交换的PH-a表示氮同位素对能带位置的影响。该发现对于利用振动光谱的苯并恶嗪的聚合研究很重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号