当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thioamide-Substituted Cinchona Alkaloids as Efficient Organocatalysts for Asymmetric Decarboxylative Reactions of MAHOs
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-08-10 03:56:30 , DOI: 10.1002/ejoc.201700870 Yuttapong Singjunla 1 , Morgane Pigeaux 1 , Romain Laporte 1 , Jérôme Baudoux 1 , Jacques Rouden 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-08-10 03:56:30 , DOI: 10.1002/ejoc.201700870 Yuttapong Singjunla 1 , Morgane Pigeaux 1 , Romain Laporte 1 , Jérôme Baudoux 1 , Jacques Rouden 1
Affiliation
|
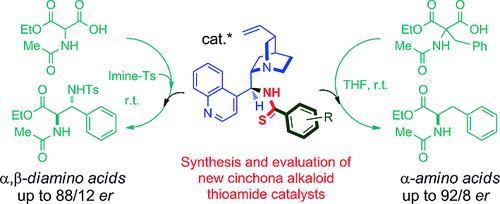
A new class of thioamide-substituted cinchona alkaloid derivatives has been successfully synthesized by an efficient approach from easily accessible dithioesters. These organocatalysts were effective in the asymmetric Mannich and enantioselective protonation reactions of α-amido-malonic acid half oxyesters (MAHOs) as cheap glycine equivalents to afford α,β- and α-amino acid derivatives, respectively.
中文翻译:

硫代酰胺取代的金鸡纳生物碱作为MAHOs不对称脱羧反应的有效有机催化剂
已经通过易于获得的二硫代酯通过有效方法成功地合成了新型的硫代酰胺取代的金鸡纳生物碱衍生物。这些有机催化剂作为廉价的甘氨酸等效物,在α-氨基丙二酸半含氧酸酯(MAHOs)的不对称曼尼希和对映选择性质子化反应中有效,分别提供α,β-和α-氨基酸衍生物。
更新日期:2017-08-10
中文翻译:

硫代酰胺取代的金鸡纳生物碱作为MAHOs不对称脱羧反应的有效有机催化剂
已经通过易于获得的二硫代酯通过有效方法成功地合成了新型的硫代酰胺取代的金鸡纳生物碱衍生物。这些有机催化剂作为廉价的甘氨酸等效物,在α-氨基丙二酸半含氧酸酯(MAHOs)的不对称曼尼希和对映选择性质子化反应中有效,分别提供α,β-和α-氨基酸衍生物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号