当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoreactions of 2-(furan-2-yl)-3-hydroxy-4H-chromen-4-one and 3-hydroxy-2-(thiophene-2-yl)-4H-chromen-4-one using cyclohexane and acetonitrile as solvents
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2017-07-13 00:00:00 , DOI: 10.1039/c7pp00106a Kulvir Kaur 1, 2, 3, 4 , Ranbir Kaur 1, 2, 3, 4 , Jyoti Tomar 1, 2, 3, 4 , Manisha Bansal 1, 2, 3, 4
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2017-07-13 00:00:00 , DOI: 10.1039/c7pp00106a Kulvir Kaur 1, 2, 3, 4 , Ranbir Kaur 1, 2, 3, 4 , Jyoti Tomar 1, 2, 3, 4 , Manisha Bansal 1, 2, 3, 4
Affiliation
|
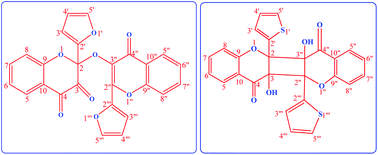
Photolysis of the titled chromenones was carried out at their longest absorption band (∼360 nm) using cyclohexane (CH) and acetonitrile (ACN) as solvents, in both aerated and de-aerated solutions. Different dimeric photoproducts were formed with both chromenones in aerated solutions. On photolysing 2-(furan-2-yl)-3-hydroxy-4H-chromen-4-one (FHC) in aerated cyclohexane, 2-(furan-2-yl)-2-{[2-(furan-2yl)-4-oxo-4H-chromen-3-yl]oxy}-2H-chromene-3,4-dione (a dehydrodimer) was formed, and on photolysing 3-hydroxy-2-(thiophene-2-yl)-4H-chromen-4-one (THC) in aerated ACN, a different dimeric product was isolated and identified. The corresponding 3-aryl-3-hydroxy-1,2-indandiones were also detected with FHC in ACN and with THC in CH, in addition to the dimeric products in both cases. On the other hand, in the de-aerated solutions, only the corresponding 1,2-indandiones were detected. 3-(Furan-2-yl)isobenzofuran-1(3H)-one as a secondary product was also detected with FHC in both solvents. An attempt was made to isolate the spectra of the photoproducts in situ. Excited State Intramolecular Proton Transfer (ESIPT) and Excited State Intramolecular Charge Transfer (ESICT) processes complicate the photodynamics of the reaction, making it difficult to predict the mechanisms of the photoreactions. However, tentative mechanisms have been proposed for the formation of the photoproducts.
中文翻译:

2-(呋喃-2-基)-3-羟基-4 H-铬烯-4-酮与3-羟基-2-(噻吩-2-基)-4 H-铬烯-4-酮的光反应使用环己烷和乙腈作为溶剂
在充气和脱气溶液中,使用环己烷(CH)和乙腈(ACN)作为溶剂,在最长的吸收带(〜360 nm)处进行标题标题的色农酮的光解。在充气溶液中,两种色酮均形成了不同的二聚光产物。在充气环己烷中光解2-(呋喃-2-基)-3-羟基-4 H-铬-4-(FHC),2-(呋喃-2-基)-2-{[2-(呋喃-形成2yl)-4-oxo-4 H -chromen-3-yl] oxy} -2 H -chromene-3,4-dione(脱氢二聚体),并在光解过程中形成3-hydroxy-2-(thiophene-2- yl)-4 H -chromen-4-one(THC)在充气的ACN中,分离并鉴定了另一种二聚体产物。除了在两种情况下的二聚体产物外,还用ACN中的FHC和CH中的THC检测到了相应的3-芳基-3-羟基-1,2-茚满二酮。另一方面,在脱气溶液中,仅检测到相应的1,2-茚满二酮。在两种溶剂中也都用FHC检测到了作为副产物的3-(呋喃-2-基)异苯并呋喃-1(3 H)-one 。试图在原位分离光产物的光谱。激发态分子内质子转移(ESIPT)和激发态分子内电荷转移(ESICT)过程使反应的光动力学复杂化,从而难以预测光反应的机理。然而,已经提出了用于形成光产物的尝试性机制。
更新日期:2017-08-09
中文翻译:

2-(呋喃-2-基)-3-羟基-4 H-铬烯-4-酮与3-羟基-2-(噻吩-2-基)-4 H-铬烯-4-酮的光反应使用环己烷和乙腈作为溶剂
在充气和脱气溶液中,使用环己烷(CH)和乙腈(ACN)作为溶剂,在最长的吸收带(〜360 nm)处进行标题标题的色农酮的光解。在充气溶液中,两种色酮均形成了不同的二聚光产物。在充气环己烷中光解2-(呋喃-2-基)-3-羟基-4 H-铬-4-(FHC),2-(呋喃-2-基)-2-{[2-(呋喃-形成2yl)-4-oxo-4 H -chromen-3-yl] oxy} -2 H -chromene-3,4-dione(脱氢二聚体),并在光解过程中形成3-hydroxy-2-(thiophene-2- yl)-4 H -chromen-4-one(THC)在充气的ACN中,分离并鉴定了另一种二聚体产物。除了在两种情况下的二聚体产物外,还用ACN中的FHC和CH中的THC检测到了相应的3-芳基-3-羟基-1,2-茚满二酮。另一方面,在脱气溶液中,仅检测到相应的1,2-茚满二酮。在两种溶剂中也都用FHC检测到了作为副产物的3-(呋喃-2-基)异苯并呋喃-1(3 H)-one 。试图在原位分离光产物的光谱。激发态分子内质子转移(ESIPT)和激发态分子内电荷转移(ESICT)过程使反应的光动力学复杂化,从而难以预测光反应的机理。然而,已经提出了用于形成光产物的尝试性机制。












































 京公网安备 11010802027423号
京公网安备 11010802027423号