当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia.
Nature Communications ( IF 14.7 ) Pub Date : 2017-08-09 , DOI: 10.1038/s41467-017-00269-1 Issaka Yougbaré , Wei-She Tai , Darko Zdravic , Brigitta Elaine Oswald , Sean Lang , Guangheng Zhu , Howard Leong-Poi , Dawei Qu , Lisa Yu , Caroline Dunk , Jianhong Zhang , John G. Sled , Stephen J. Lye , Jelena Brkić , Chun Peng , Petter Höglund , B. Anne Croy , S. Lee Adamson , Xiao-Yan Wen , Duncan J. Stewart , John Freedman , Heyu Ni
Nature Communications ( IF 14.7 ) Pub Date : 2017-08-09 , DOI: 10.1038/s41467-017-00269-1 Issaka Yougbaré , Wei-She Tai , Darko Zdravic , Brigitta Elaine Oswald , Sean Lang , Guangheng Zhu , Howard Leong-Poi , Dawei Qu , Lisa Yu , Caroline Dunk , Jianhong Zhang , John G. Sled , Stephen J. Lye , Jelena Brkić , Chun Peng , Petter Höglund , B. Anne Croy , S. Lee Adamson , Xiao-Yan Wen , Duncan J. Stewart , John Freedman , Heyu Ni
|
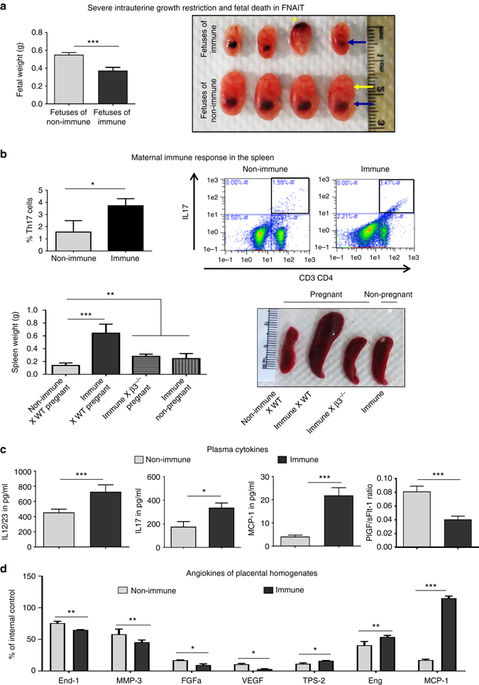
Miscarriage and intrauterine growth restriction (IUGR) are devastating complications in fetal/neonatal alloimmune thrombocytopenia (FNAIT). We previously reported the mechanisms for bleeding diatheses, but it is unknown whether placental, decidual immune cells or other abnormalities at the maternal-fetal interface contribute to FNAIT. Here we show that maternal immune responses to fetal platelet antigens cause miscarriage and IUGR that are associated with vascular and immune pathologies in murine FNAIT models. Uterine natural killer (uNK) cell recruitment and survival beyond mid-gestation lead to elevated NKp46 and CD107 expression, perforin release and trophoblast apoptosis. Depletion of NK cells restores normal spiral artery remodeling and placental function, prevents miscarriage, and rescues hemorrhage in neonates. Blockade of NK activation receptors (NKp46, FcɣRIIIa) also rescues pregnancy loss. These findings shed light on uNK antibody-dependent cell-mediated cytotoxicity of invasive trophoblasts as a pathological mechanism in FNAIT, and suggest that anti-NK cell therapies may prevent immune-mediated pregnancy loss and ameliorate FNAIT.Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is a gestational disease caused by maternal immune responses against fetal platelets. Using a FNAIT mouse model and human trophoblast cell lines, here the authors show that uterine natural killer cell-mediated trophoblast apoptosis contributes to FNAIT pathogenesis.
中文翻译:

活化的NK细胞会引起胎盘功能障碍,并在胎儿同种免疫性血小板减少症中流产。
流产和宫内生长受限(IUGR)是胎儿/新生儿同种免疫血小板减少症(FNAIT)的毁灭性并发症。我们先前曾报道过出血性血液透析的机制,但尚不清楚胎盘,蜕膜免疫细胞或母胎界面的其他异常是否有助于FNAIT。在这里,我们显示母体对胎儿血小板抗原的免疫反应会导致流产和IUGR,这与鼠类FNAIT模型中的血管和免疫病理有关。子宫自然杀伤(uNK)细胞募集和存活超过妊娠中期会导致NKp46和CD107表达升高,穿孔素释放和滋养细胞凋亡。NK细胞耗竭可恢复正常的螺旋动脉重塑和胎盘功能,防止流产,并挽救新生儿的出血。NK激活受体(NKp46,FcɣRIIIa)的阻滞也可以挽救妊娠。这些发现阐明了侵袭性滋养细胞的uNK抗体依赖性细胞介导的细胞毒性是FNAIT的病理机制,并表明抗NK细胞疗法可预防免疫介导的妊娠流失并改善FNAIT。胎儿/新生儿同种异体免疫性血小板减少症(FNAIT)是由孕妇针对胎儿血小板的免疫反应引起的妊娠疾病。作者使用FNAIT小鼠模型和人类滋养层细胞系,显示子宫自然杀伤细胞介导的滋养层细胞凋亡促成FNAIT发病机理。提示抗NK细胞疗法可预防免疫介导的妊娠丢失并改善FNAIT。胎儿/新生儿同种免疫性血小板减少症(FNAIT)是由孕妇对胎儿血小板的免疫反应引起的妊娠疾病。作者使用FNAIT小鼠模型和人类滋养层细胞系,显示子宫自然杀伤细胞介导的滋养层细胞凋亡促成FNAIT发病机理。提示抗NK细胞疗法可预防免疫介导的妊娠丢失并改善FNAIT。胎儿/新生儿同种免疫性血小板减少症(FNAIT)是由孕妇对胎儿血小板的免疫反应引起的妊娠疾病。作者使用FNAIT小鼠模型和人类滋养层细胞系,显示子宫自然杀伤细胞介导的滋养层细胞凋亡促成FNAIT发病机理。
更新日期:2017-08-09
中文翻译:

活化的NK细胞会引起胎盘功能障碍,并在胎儿同种免疫性血小板减少症中流产。
流产和宫内生长受限(IUGR)是胎儿/新生儿同种免疫血小板减少症(FNAIT)的毁灭性并发症。我们先前曾报道过出血性血液透析的机制,但尚不清楚胎盘,蜕膜免疫细胞或母胎界面的其他异常是否有助于FNAIT。在这里,我们显示母体对胎儿血小板抗原的免疫反应会导致流产和IUGR,这与鼠类FNAIT模型中的血管和免疫病理有关。子宫自然杀伤(uNK)细胞募集和存活超过妊娠中期会导致NKp46和CD107表达升高,穿孔素释放和滋养细胞凋亡。NK细胞耗竭可恢复正常的螺旋动脉重塑和胎盘功能,防止流产,并挽救新生儿的出血。NK激活受体(NKp46,FcɣRIIIa)的阻滞也可以挽救妊娠。这些发现阐明了侵袭性滋养细胞的uNK抗体依赖性细胞介导的细胞毒性是FNAIT的病理机制,并表明抗NK细胞疗法可预防免疫介导的妊娠流失并改善FNAIT。胎儿/新生儿同种异体免疫性血小板减少症(FNAIT)是由孕妇针对胎儿血小板的免疫反应引起的妊娠疾病。作者使用FNAIT小鼠模型和人类滋养层细胞系,显示子宫自然杀伤细胞介导的滋养层细胞凋亡促成FNAIT发病机理。提示抗NK细胞疗法可预防免疫介导的妊娠丢失并改善FNAIT。胎儿/新生儿同种免疫性血小板减少症(FNAIT)是由孕妇对胎儿血小板的免疫反应引起的妊娠疾病。作者使用FNAIT小鼠模型和人类滋养层细胞系,显示子宫自然杀伤细胞介导的滋养层细胞凋亡促成FNAIT发病机理。提示抗NK细胞疗法可预防免疫介导的妊娠丢失并改善FNAIT。胎儿/新生儿同种免疫性血小板减少症(FNAIT)是由孕妇对胎儿血小板的免疫反应引起的妊娠疾病。作者使用FNAIT小鼠模型和人类滋养层细胞系,显示子宫自然杀伤细胞介导的滋养层细胞凋亡促成FNAIT发病机理。






























 京公网安备 11010802027423号
京公网安备 11010802027423号