当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoradiation Cancer Therapy: Molecular Mechanisms of Cisplatin Radiosensitization
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/acs.jpcc.7b05271 Yanfang Dong 1 , Limei Zhou 1 , Qinfen Tian 1 , Yi Zheng 1 , Léon Sanche 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/acs.jpcc.7b05271 Yanfang Dong 1 , Limei Zhou 1 , Qinfen Tian 1 , Yi Zheng 1 , Léon Sanche 2
Affiliation
|
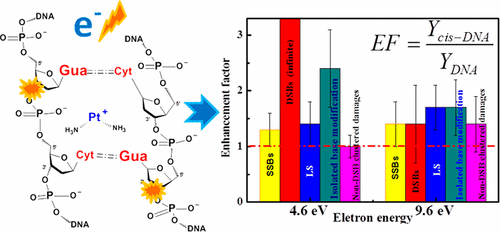
Sensitization of malignant cells to ionizing radiation plays a key role in cancer treatments that combine chemotherapy and radiation therapy. Enhancement by chemotherapeutic agents of DNA base damages and clustered damage, which are among the most detrimental cellular modifications, is expected to contribute significantly to the radiosensitization process; however, with the exception of double-strand breaks (DSBs), no measurements exist on the enhancement of such damages induced by the abundant secondary low-energy electrons (LEEs) created by ionizing radiation. This lack of information restricts our global understanding of the molecular mechanisms involved in chemoradiation therapy. Here we measure the enhancements of LEE-induced damages resulting from the binding of cisplatin to plasmid DNA (pGEM-3Zf(−), 3197 bp). The enhancement factors (EFs) are reported for base damages (BDs) on one strand and clustered damages consisting of BDs and single-strand break (SSBs) with adjacent BDs on the opposite strand. Five-monolayer films of cisplatin–plasmid-DNA complexes in a molar ratio of 5:1 are prepared by lyophilization and irradiated in vacuum with monoenergetic electrons at the resonance energies of 4.6 and 9.6 eV. Cross-links, SSBs, DSBs and the loss of the supercoiled configuration are analyzed by the agarose gel electrophoresis. Irradiated samples are treated with E. coli base excision repair endonuclease (Nth and Fpg) enzymes to reveal by electrophoresis base modifications occurring within two helical turns of the DNA helix. From the dose–response curves, the total DNA damages induced at each energy are 244 ± 42 and 359 ± 44 × 10–15 electron–1 molecule–1, while the percentages of base modifications in these totals are 55 and 54%, respectively. Binding of cisplatin to DNA increases base modifications by EFs of 2.4 and 1.9, respectively; these are the largest finite EFs observed. Enhancement of all lesions can be explained by invoking two general mechanisms of electron transfer coupled to transient anions formation in cisplatin–DNA complexes. The present results provide more complete information on the radiosensitization of LEE-induced damages to DNA by cisplatin.
中文翻译:

化学放射疗法:顺铂放射敏化的分子机制
在结合化学疗法和放射疗法的癌症治疗中,恶性细胞对电离辐射的敏感性起着关键作用。化疗药物对DNA碱基损伤和簇状损伤的损害是最有害的细胞修饰之一,有望大大促进放射增敏过程;但是,除了双链断裂(DSB)以外,没有任何方法可以测量由电离辐射产生的大量次级低能电子(LEE)引起的这种损伤的增强。信息的缺乏限制了我们对化学放射治疗所涉及的分子机制的整体理解。在这里,我们测量了由顺铂与质粒DNA(pGEM-3Zf(-),3197 bp)的结合导致的LEE诱导的损伤的增强。报告增强因子(EFs)表示单链上的碱基损伤(BD)和聚类损伤,包括BDs和单链断裂(SSB)以及相反链上的相邻BD。通过冷冻干燥制备摩尔比为5:1的顺铂-质粒-DNA复合物的五层单层膜,并在真空中用单能电子以4.6和9.6 eV的共振能量进行辐照。通过琼脂糖凝胶电泳分析交联,SSB,DSB和超螺旋构型的损失。辐照样品用 通过冻干制备图1的样品,并在真空中用单能电子以4.6和9.6eV的共振能量照射。通过琼脂糖凝胶电泳分析交联,SSB,DSB和超螺旋构型的损失。辐照样品用 通过冻干制备图1的样品,并在真空中用单能电子以4.6和9.6eV的共振能量照射。通过琼脂糖凝胶电泳分析交联,SSB,DSB和超螺旋构型的损失。辐照样品用大肠杆菌碱基切除修复核酸内切酶(Nth和Fpg),通过电泳揭示DNA螺旋的两个螺旋圈内发生的碱基修饰。从剂量反应曲线,每种能量引起的总DNA损伤为244±42和359±44×10 –15电子–1分子–1,而碱基修饰在这些总数中所占的百分比分别为55%和54%。顺铂与DNA的结合分别增加了2.4和1.9的EF引起的碱基修饰;这些是观察到的最大有限EF。可以通过调用两种电子转移的一般机理来解释所有病变的增强,这些机理与顺铂-DNA复合物中的瞬时阴离子形成有关。目前的结果提供了关于顺铂对LEE诱导的DNA损伤的放射增敏的更完整的信息。
更新日期:2017-08-09
中文翻译:

化学放射疗法:顺铂放射敏化的分子机制
在结合化学疗法和放射疗法的癌症治疗中,恶性细胞对电离辐射的敏感性起着关键作用。化疗药物对DNA碱基损伤和簇状损伤的损害是最有害的细胞修饰之一,有望大大促进放射增敏过程;但是,除了双链断裂(DSB)以外,没有任何方法可以测量由电离辐射产生的大量次级低能电子(LEE)引起的这种损伤的增强。信息的缺乏限制了我们对化学放射治疗所涉及的分子机制的整体理解。在这里,我们测量了由顺铂与质粒DNA(pGEM-3Zf(-),3197 bp)的结合导致的LEE诱导的损伤的增强。报告增强因子(EFs)表示单链上的碱基损伤(BD)和聚类损伤,包括BDs和单链断裂(SSB)以及相反链上的相邻BD。通过冷冻干燥制备摩尔比为5:1的顺铂-质粒-DNA复合物的五层单层膜,并在真空中用单能电子以4.6和9.6 eV的共振能量进行辐照。通过琼脂糖凝胶电泳分析交联,SSB,DSB和超螺旋构型的损失。辐照样品用 通过冻干制备图1的样品,并在真空中用单能电子以4.6和9.6eV的共振能量照射。通过琼脂糖凝胶电泳分析交联,SSB,DSB和超螺旋构型的损失。辐照样品用 通过冻干制备图1的样品,并在真空中用单能电子以4.6和9.6eV的共振能量照射。通过琼脂糖凝胶电泳分析交联,SSB,DSB和超螺旋构型的损失。辐照样品用大肠杆菌碱基切除修复核酸内切酶(Nth和Fpg),通过电泳揭示DNA螺旋的两个螺旋圈内发生的碱基修饰。从剂量反应曲线,每种能量引起的总DNA损伤为244±42和359±44×10 –15电子–1分子–1,而碱基修饰在这些总数中所占的百分比分别为55%和54%。顺铂与DNA的结合分别增加了2.4和1.9的EF引起的碱基修饰;这些是观察到的最大有限EF。可以通过调用两种电子转移的一般机理来解释所有病变的增强,这些机理与顺铂-DNA复合物中的瞬时阴离子形成有关。目前的结果提供了关于顺铂对LEE诱导的DNA损伤的放射增敏的更完整的信息。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号