当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal-Free Decarboxylative Iodination: New Routes for Decarboxylative Oxidative Cross-Couplings
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/jacs.7b05155
Gregory J. P. Perry 1 , Jacob M. Quibell 1 , Adyasha Panigrahi 1 , Igor Larrosa 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1021/jacs.7b05155
Gregory J. P. Perry 1 , Jacob M. Quibell 1 , Adyasha Panigrahi 1 , Igor Larrosa 1
Affiliation
|
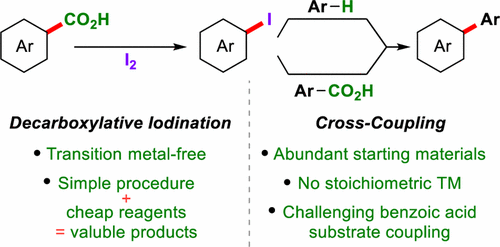
Constructing products of high synthetic value from inexpensive and abundant starting materials is of great importance. Aryl iodides are essential building blocks for the synthesis of functional molecules, and efficient methods for their synthesis from chemical feedstocks are highly sought after. Here we report a low-cost decarboxylative iodination that occurs simply from readily available benzoic acids and I2. The reaction is scalable and the scope and robustness of the reaction is thoroughly examined. Mechanistic studies suggest that this reaction does not proceed via a radical mechanism, which is in contrast to classical Hunsdiecker-type decarboxylative halogenations. In addition, DFT studies allow comparisons to be made between our procedure and current transition-metal-catalyzed decarboxylations. The utility of this procedure is demonstrated in its application to oxidative cross-couplings of aromatics via decarboxylative/C–H or double decarboxylative activations that use I2 as the terminal oxidant. This strategy allows the preparation of biaryls previously inaccessible via decarboxylative methods and holds other advantages over existing decarboxylative oxidative couplings, as stoichiometric transition metals are avoided.
中文翻译:

无过渡金属的脱羧碘化:脱羧氧化交叉偶联的新途径。
由廉价和丰富的起始原料构建具有高合成价值的产品非常重要。芳基碘化物是合成功能分子的重要组成部分,并且高度寻求从化学原料合成它们的有效方法。在这里,我们报告了一种低成本的脱羧碘化反应,该反应仅是由容易获得的苯甲酸和I 2发生的。。该反应是可扩展的,并且已彻底检查了反应的范围和稳健性。机理研究表明,该反应不是通过自由基机理进行的,这与经典的Hunsdiecker型脱羧卤化反应相反。另外,DFT研究允许在我们的方法与当前过渡金属催化的脱羧反应之间进行比较。该方法的实用性在通过I 2的脱羧/ CH或双脱羧活化反应应用于芳族化合物的氧化交叉偶联中得到了证明。作为末端氧化剂。该策略允许制备先前无法通过脱羧方法获得的联芳基,并且由于避免了化学计量的过渡金属,因此与现有的脱羧氧化偶联相比具有其他优点。
更新日期:2017-08-09
中文翻译:

无过渡金属的脱羧碘化:脱羧氧化交叉偶联的新途径。
由廉价和丰富的起始原料构建具有高合成价值的产品非常重要。芳基碘化物是合成功能分子的重要组成部分,并且高度寻求从化学原料合成它们的有效方法。在这里,我们报告了一种低成本的脱羧碘化反应,该反应仅是由容易获得的苯甲酸和I 2发生的。。该反应是可扩展的,并且已彻底检查了反应的范围和稳健性。机理研究表明,该反应不是通过自由基机理进行的,这与经典的Hunsdiecker型脱羧卤化反应相反。另外,DFT研究允许在我们的方法与当前过渡金属催化的脱羧反应之间进行比较。该方法的实用性在通过I 2的脱羧/ CH或双脱羧活化反应应用于芳族化合物的氧化交叉偶联中得到了证明。作为末端氧化剂。该策略允许制备先前无法通过脱羧方法获得的联芳基,并且由于避免了化学计量的过渡金属,因此与现有的脱羧氧化偶联相比具有其他优点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号