当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycoclusters as lectin inhibitors: comparative analysis on two plant agglutinins with different folding as a step towards rules for selectivity
Tetrahedron ( IF 2.1 ) Pub Date : 2015-08-10 11:25:12 Sabine André, Shane O'Sullivan, Hans-Joachim Gabius, Paul V. Murphy
Tetrahedron ( IF 2.1 ) Pub Date : 2015-08-10 11:25:12 Sabine André, Shane O'Sullivan, Hans-Joachim Gabius, Paul V. Murphy
|
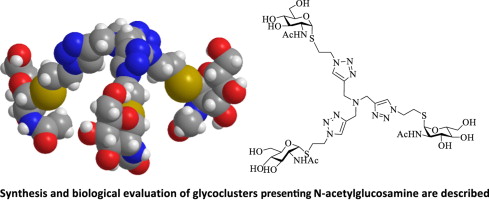
The emerging physiological significance of carbohydrate (glycan)–protein (lectin) recognition engenders the interest to design synthetic inhibitors with a high level of selectivity among natural sugar receptors. Plant agglutinins are common models to determine structure–activity relationships. Focussing on the contribution of valency towards selectivity, copper-catalysed azide (sugar derivative)–alkyne (scaffold) cycloaddition yielded a panel of 10 bi- to tetravalent glycoclusters with N-acetylglucosamine as the bioactive headgroup. They were introduced into assays using (neo)glycoproteins and cell surfaces as platforms to study carbohydrate-dependent lectin binding. The ability of the bivalent compounds, which exhibit a distance profile of the sugar headgroups of about 16–21 Å, for intramolecular bridging of two contact sites from the eight hevein domains of wheat germ agglutinin led to comparatively high enhancements of inhibitory potency relative to a tetrameric leguminous lectin (distance profile of 50–70 Å between sugar-specific sites), especially for a β-S-glycoside. The extent of inhibition at fixed concentrations of the sugar depended on the type of matrix used for the assay. Increases to tri- and tetravalency played a less important role than the anomeric position to keep cross-reactivity low, these tested topologies enabling cross-linking for both lectins. The potential for cis-interactions (intramolecular interactions), with glycoclusters serving as molecular rulers, is suggested to help designing selective blocking reagents.
中文翻译:

糖簇作为凝集素抑制剂:对两种植物凝集素的不同折叠进行比较分析,这是朝着选择性规则迈进的一步
碳水化合物(聚糖)-蛋白质(凝集素)识别的新兴生理意义引起了人们的兴趣,即设计一种在天然糖受体之间具有高选择性的合成抑制剂。植物凝集素是确定结构-活性关系的常用模型。着眼于化合价对选择性的贡献,铜催化的叠氮化物(糖衍生物)-炔烃(骨架)环加成反应产生了一组10个二价至四价糖团,其中N-乙酰氨基葡萄糖为生物活性头基。将它们引入使用(新)糖蛋白和细胞表面作为平台的测定法中,以研究碳水化合物依赖性凝集素的结合。二价化合物的能力,其糖首基的距离分布约为16-21Å,小麦胚芽凝集素的八个hevein结构域中两个接触点的分子内桥接导致相对于四聚体豆科植物凝集素(糖特异性位点之间的距离分布为50-70Å),抑制效力的提高相对较高,尤其是对于β- S-糖苷。在糖的固定浓度下的抑制程度取决于用于测定的基质的类型。三价和四价的增加在保持低交叉反应性方面不如端基异构体重要,这些经过测试的拓扑结构可实现两种凝集素的交联。建议以糖团作为分子标尺进行顺式相互作用(分子间相互作用)的潜力,以帮助设计选择性封闭剂。
更新日期:2015-08-11
中文翻译:

糖簇作为凝集素抑制剂:对两种植物凝集素的不同折叠进行比较分析,这是朝着选择性规则迈进的一步
碳水化合物(聚糖)-蛋白质(凝集素)识别的新兴生理意义引起了人们的兴趣,即设计一种在天然糖受体之间具有高选择性的合成抑制剂。植物凝集素是确定结构-活性关系的常用模型。着眼于化合价对选择性的贡献,铜催化的叠氮化物(糖衍生物)-炔烃(骨架)环加成反应产生了一组10个二价至四价糖团,其中N-乙酰氨基葡萄糖为生物活性头基。将它们引入使用(新)糖蛋白和细胞表面作为平台的测定法中,以研究碳水化合物依赖性凝集素的结合。二价化合物的能力,其糖首基的距离分布约为16-21Å,小麦胚芽凝集素的八个hevein结构域中两个接触点的分子内桥接导致相对于四聚体豆科植物凝集素(糖特异性位点之间的距离分布为50-70Å),抑制效力的提高相对较高,尤其是对于β- S-糖苷。在糖的固定浓度下的抑制程度取决于用于测定的基质的类型。三价和四价的增加在保持低交叉反应性方面不如端基异构体重要,这些经过测试的拓扑结构可实现两种凝集素的交联。建议以糖团作为分子标尺进行顺式相互作用(分子间相互作用)的潜力,以帮助设计选择性封闭剂。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号