European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-08-07 , DOI: 10.1016/j.ejmech.2017.08.018 Jianguo Qi , Haiyang Dong , Jing Huang , Shufeng Zhang , Linqiang Niu , Yahong Zhang , Jianhong Wang
|
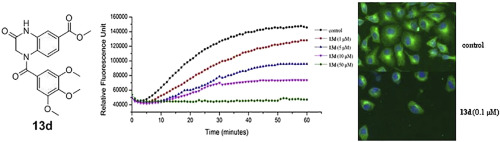
A series of novel N-substituted 3-oxo-1,2,3,4-tetrahydro-quinoxaline-6-carboxy- lic acid derivatives were synthesized and evaluated for their biological activities. Among all synthesized target compounds, 13d exhibited the most potent antiproliferative activity against HeLa, SMMC-7721, K562 cell line (IC50 = 0.126 μM, 0.071 μM, 0.164 μM, respectively). Furthermore, compound 13d inhibited tubulin polymerization (IC50 = 3.97 μM), arrested cell cycle at the G2/M phase and induced apoptosis. The binding mode at the colchicine binding site was also probed. These studies provided a new molecular scaffold for the further development of antitumor agents that target tubulin.
中文翻译:

微管蛋白聚合抑制剂N-取代的3-氧代-1,2,3,4-四氢-喹喔啉-6-羧酸衍生物的合成及生物学评价
合成了一系列新颖的N-取代的3-氧代-1,2,3,4-四氢-喹喔啉-6-羧酸衍生物,并对其生物学活性进行了评估。在所有合成的目标化合物中,13d对HeLa,SMMC-7721,K562细胞系表现出最有效的抗增殖活性(IC 50分别 为0.126μM,0.071μM,0.164μM)。此外,化合物13d抑制微管蛋白聚合(IC 50 = 3.97μM),使细胞周期停滞在G2 / M期并诱导凋亡。还探讨了秋水仙碱结合位点的结合方式。这些研究为靶向微管蛋白的抗肿瘤药物的进一步开发提供了新的分子支架。






























 京公网安备 11010802027423号
京公网安备 11010802027423号