当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CuCl-Catalyzed Hydroxylation of N-Heteroarylcarbazole Bromide: Approach for the Preparation of N-Heteroarylcarbazolyl Phenols and Its Application in the Synthesis of Phosphorescent Emitters
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/acs.joc.7b01568 Guijie Li 1 , Xiangdong Zhao 1 , Kun Fang 1 , Jian Li 2 , Yuanbin She 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/acs.joc.7b01568 Guijie Li 1 , Xiangdong Zhao 1 , Kun Fang 1 , Jian Li 2 , Yuanbin She 1
Affiliation
|
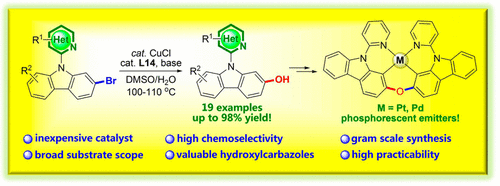
An efficient and practical CuCl-catalyzed hydroxylation of N-heteroarylcarbazole bromide for the preparation of N-heteroarylcarbazolyl phenols with a broad functional group scope and yield up to 98% was developed. It was found that both the ligand and base played critical roles in the functional group transformation and that different products could be generated by changing the base for some substrates. t-BuONa was demonstrated to be a better base for the catalytic system to avoid the formation of the ether byproduct. In addition, this approach was suitable for large-scale preparation and was successfully applied in the gram-scale synthesis of phosphorescent emitters PtNON and PdNON, demonstrating its practicability in organic synthesis methodology and materials science. Furthermore, the X-ray crystal diffraction, DFT calculations, and photophysical properties were also investigated for the metal complexes.
中文翻译:

CuCl催化的N-杂芳基咔唑溴化物的羟基化反应:N-杂芳基咔唑基苯酚的制备方法及其在磷光发射体合成中的应用
开发了一种高效,实用的CuCl催化的N-杂芳基咔唑溴化物羟基化反应,用于制备官能团范围广,收率高达98%的N-杂芳基咔唑基酚。发现配体和碱基都在官能团转化中起关键作用,并且通过改变某些底物的碱基可以产生不同的产物。已证明t- BuONa是催化体系避免醚副产物形成的更好的碱。此外,该方法适用于大规模制备,并成功地用于克级合成的磷光发射体PtNON和PdNON,证明了其在有机合成方法和材料科学中的实用性。此外,还研究了金属配合物的X射线晶体衍射,DFT计算和光物理性质。
更新日期:2017-08-07
中文翻译:

CuCl催化的N-杂芳基咔唑溴化物的羟基化反应:N-杂芳基咔唑基苯酚的制备方法及其在磷光发射体合成中的应用
开发了一种高效,实用的CuCl催化的N-杂芳基咔唑溴化物羟基化反应,用于制备官能团范围广,收率高达98%的N-杂芳基咔唑基酚。发现配体和碱基都在官能团转化中起关键作用,并且通过改变某些底物的碱基可以产生不同的产物。已证明t- BuONa是催化体系避免醚副产物形成的更好的碱。此外,该方法适用于大规模制备,并成功地用于克级合成的磷光发射体PtNON和PdNON,证明了其在有机合成方法和材料科学中的实用性。此外,还研究了金属配合物的X射线晶体衍射,DFT计算和光物理性质。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号