当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of a Bicyclic Azetidine with In Vivo Antimalarial Activity Enabled by Stereospecific, Directed C(sp3)–H Arylation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/jacs.7b06994 Micah Maetani 1, 2 , Jochen Zoller 1, 2 , Bruno Melillo 1, 2 , Oscar Verho 1, 2 , Nobutaka Kato 2 , Jun Pu 2 , Eamon Comer 2 , Stuart L. Schreiber 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-07 00:00:00 , DOI: 10.1021/jacs.7b06994 Micah Maetani 1, 2 , Jochen Zoller 1, 2 , Bruno Melillo 1, 2 , Oscar Verho 1, 2 , Nobutaka Kato 2 , Jun Pu 2 , Eamon Comer 2 , Stuart L. Schreiber 1, 2, 3
Affiliation
|
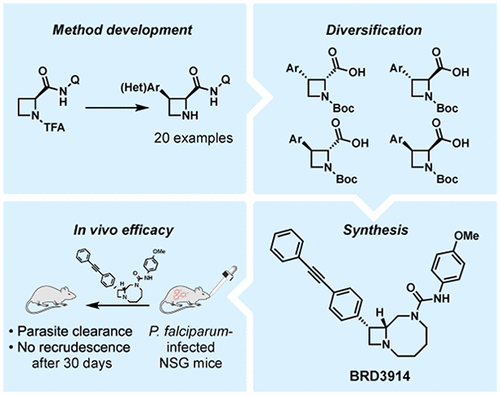
The development of new antimalarial therapeutics is necessary to address the increasing resistance to current drugs. Bicyclic azetidines targeting Plasmodium falciparum phenylalanyl-tRNA synthetase comprise one promising new class of antimalarials, especially due to their activities against three stages of the parasite’s life cycle, but a lengthy synthetic route to these compounds may affect the feasibility of delivering new therapeutic agents within the cost constraints of antimalarial drugs. Here, we report an efficient synthesis of antimalarial compound BRD3914 (EC50 = 15 nM) that hinges on a Pd-catalyzed, directed C(sp3)–H arylation of azetidines at the C3 position. This newly developed protocol exhibits a broad substrate scope and provides access to valuable, stereochemically defined building blocks. BRD3914 was evaluated in P. falciparum-infected mice, providing a cure after four oral doses.
中文翻译:

立体定向C(sp 3)–H芳基化使具有抗疟活性的双环氮杂环丁烷的合成
开发新的抗疟疾疗法对解决当前对药物日益增加的耐药性是必要的。靶向恶性疟原虫苯丙氨酰-tRNA合成酶的双环氮杂环丁烷包含一类有前途的新型抗疟药,特别是由于它们对寄生虫生命周期的三个阶段具有活性,但是合成这些化合物的漫长路线可能会影响在其体内递送新治疗剂的可行性。抗疟药的成本限制。在这里,我们报告了抗疟疾化合物BRD3914(EC 50 = 15 nM)的有效合成,该化合物依赖于Pd催化的定向C(sp 3)–氮杂环丁烷在C3位的H芳基化。该新开发的协议具有广阔的底物范围,并提供了有价值的,立体化学定义的构建基块。在恶性疟原虫感染的小鼠中评估了BRD3914 ,在口服四次后即可治愈。
更新日期:2017-08-07
中文翻译:

立体定向C(sp 3)–H芳基化使具有抗疟活性的双环氮杂环丁烷的合成
开发新的抗疟疾疗法对解决当前对药物日益增加的耐药性是必要的。靶向恶性疟原虫苯丙氨酰-tRNA合成酶的双环氮杂环丁烷包含一类有前途的新型抗疟药,特别是由于它们对寄生虫生命周期的三个阶段具有活性,但是合成这些化合物的漫长路线可能会影响在其体内递送新治疗剂的可行性。抗疟药的成本限制。在这里,我们报告了抗疟疾化合物BRD3914(EC 50 = 15 nM)的有效合成,该化合物依赖于Pd催化的定向C(sp 3)–氮杂环丁烷在C3位的H芳基化。该新开发的协议具有广阔的底物范围,并提供了有价值的,立体化学定义的构建基块。在恶性疟原虫感染的小鼠中评估了BRD3914 ,在口服四次后即可治愈。































 京公网安备 11010802027423号
京公网安备 11010802027423号