当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Spin Iron Imido Complexes Competent for C–H Bond Amination
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-18 , DOI: 10.1021/jacs.7b06682 Matthew J T Wilding 1 , Diana A Iovan 1 , Theodore A Betley 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-18 , DOI: 10.1021/jacs.7b06682 Matthew J T Wilding 1 , Diana A Iovan 1 , Theodore A Betley 1
Affiliation
|
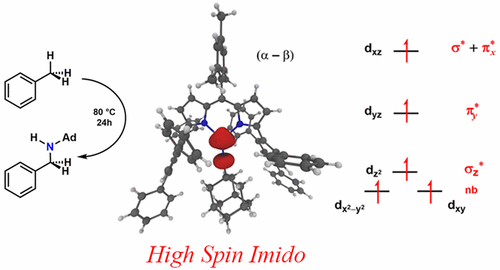
Reduction of previously reported (ArL)FeCl with potassium graphite furnished a low-spin (S = 1/2) iron complex (ArL)Fe which features an intramolecular η6-arene interaction and can be utilized as an FeI synthon (ArL = 5-mesityl-1,9-(2,4,6-Ph3C6H2)dipyrrin). Treatment of (ArL)Fe with adamantyl azide or mesityl azide led to the formation of the high-spin (S = 5/2), three-coordinate imidos (ArL)Fe(NAd) and (ArL)Fe(NMes), respectively, as determined by EPR, zero-field 57Fe Mössbauer, magnetometry, and single crystal X-ray diffraction. The high-spin iron imidos are reactive with a variety of substrates: (ArL)Fe(NAd) reacts with azide yielding a ferrous tetrazido (ArL)Fe(κ2-N4Ad2), undergoes intermolecular nitrene transfer to phosphine, abstracts H atoms from weak C-H bonds (1,4-cyclohexadiene, 2,4,6-tBu3C6H2OH) to afford ferrous amido product (ArL)Fe(NHAd), and can mediate intermolecular C-H amination of toluene [PhCH3/PhCD3 kH/kD: 15.5(3); PhCH2D kH/kD: 11(1)]. The C-H bond functionalization reactivity is rationalized from a two-step mechanism wherein each step occurs via maximal energy and orbital overlap between the imido fragment and the C-H bond containing substrate.
中文翻译:

能够进行 C-H 键胺化的高自旋亚氨基铁络合物
用钾石墨还原先前报道的 (ArL)FeCl 提供了低自旋 (S = 1/2) 铁配合物 (ArL)Fe,其具有分子内 η6-芳烃相互作用,可用作 FeI 合成子 (ArL = 5-异丙叉基-1,9-(2,4,6-Ph3C6H2)二吡林)。用金刚烷基叠氮化物或异丙基叠氮化物处理 (ArL)Fe 分别导致高自旋 (S = 5/2)、三配位亚氨基 (ArL)Fe(NAd) 和 (ArL)Fe(NMes) 的形成,通过 EPR、零场 57Fe 穆斯堡尔、磁力测定和单晶 X 射线衍射测定。高自旋铁亚胺基可与多种底物发生反应:(ArL)Fe(NAd) 与叠氮化物反应生成四叠氮亚铁 (ArL)Fe(κ2-N4Ad2),经历分子间氮烯转移为膦,从弱原子中提取 H 原子CH 键合 (1,4-环己二烯, 2,4,6-tBu3C6H2OH) 生成亚铁酰氨基产物 (ArL)Fe(NHAd),并且可以介导甲苯的分子间 CH 胺化 [PhCH3/PhCD3 kH/kD: 15.5(3) ; PhCH2D kH/kD:11(1)]。 CH键官能化反应性由两步机制合理化,其中每个步骤通过酰亚胺片段和含有CH键的底物之间的最大能量和轨道重叠发生。
更新日期:2017-08-18
中文翻译:

能够进行 C-H 键胺化的高自旋亚氨基铁络合物
用钾石墨还原先前报道的 (ArL)FeCl 提供了低自旋 (S = 1/2) 铁配合物 (ArL)Fe,其具有分子内 η6-芳烃相互作用,可用作 FeI 合成子 (ArL = 5-异丙叉基-1,9-(2,4,6-Ph3C6H2)二吡林)。用金刚烷基叠氮化物或异丙基叠氮化物处理 (ArL)Fe 分别导致高自旋 (S = 5/2)、三配位亚氨基 (ArL)Fe(NAd) 和 (ArL)Fe(NMes) 的形成,通过 EPR、零场 57Fe 穆斯堡尔、磁力测定和单晶 X 射线衍射测定。高自旋铁亚胺基可与多种底物发生反应:(ArL)Fe(NAd) 与叠氮化物反应生成四叠氮亚铁 (ArL)Fe(κ2-N4Ad2),经历分子间氮烯转移为膦,从弱原子中提取 H 原子CH 键合 (1,4-环己二烯, 2,4,6-tBu3C6H2OH) 生成亚铁酰氨基产物 (ArL)Fe(NHAd),并且可以介导甲苯的分子间 CH 胺化 [PhCH3/PhCD3 kH/kD: 15.5(3) ; PhCH2D kH/kD:11(1)]。 CH键官能化反应性由两步机制合理化,其中每个步骤通过酰亚胺片段和含有CH键的底物之间的最大能量和轨道重叠发生。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号