当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acyclic 1,4-Stereocontrol via the Allylic Diazene Rearrangement: Development, Applications, and the Essential Role of Kinetic E Stereoselectivity in Tosylhydrazone Formation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-08-04 00:00:00 , DOI: 10.1021/acs.joc.7b00428 Maha L. Shrestha 1 , Wei Qi 1 , Matthias C. McIntosh 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-08-04 00:00:00 , DOI: 10.1021/acs.joc.7b00428 Maha L. Shrestha 1 , Wei Qi 1 , Matthias C. McIntosh 1
Affiliation
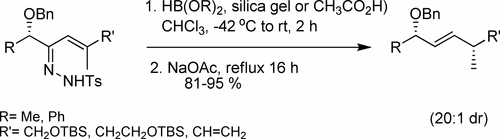
|
We report full details of a method for 1,3-reductive transposition of α-alkoxy-α,β-unsaturated hydrazones to provide E-alkenes with high 1,4-stereocontrol between the two respective allylic stereocenters. The process couples a chelation-controlled reduction of the hydrazone with an in situ allylic strain controlled retro-ene reaction of an allyl diazene, i.e., an allylic diazene rearrangement. Such stereotriads are frequently observed motifs in natural products. We observed a fortuitous kinetic preference for the E-hydrazone geometry during the hydrazonation reaction, as only the E-isomers could undergo chelation-controlled reduction.
中文翻译:

通过烯丙基二氮烯重排进行非环1,4-立体控制:甲苯磺酰Formation形成中动力学E立体选择性的发展,应用和基本作用
我们报告了α-烷氧基-α,β-不饱和的1,3-还原转座的方法的全部细节,以提供在两个各自的烯丙基立体中心之间具有高1,4-立体控制的E-烯烃。该方法将的螯合控制的还原与烯丙基二氮烯的原位烯丙基应变控制的逆向烯反应偶联,即烯丙基二氮烯重排。这种立体三脚架是天然产物中经常观察到的基序。我们观察到在化反应期间对E- hydr几何形状的偶然动力学偏好,因为只有E-异构体可以进行螯合控制的还原。
更新日期:2017-08-04
中文翻译:

通过烯丙基二氮烯重排进行非环1,4-立体控制:甲苯磺酰Formation形成中动力学E立体选择性的发展,应用和基本作用
我们报告了α-烷氧基-α,β-不饱和的1,3-还原转座的方法的全部细节,以提供在两个各自的烯丙基立体中心之间具有高1,4-立体控制的E-烯烃。该方法将的螯合控制的还原与烯丙基二氮烯的原位烯丙基应变控制的逆向烯反应偶联,即烯丙基二氮烯重排。这种立体三脚架是天然产物中经常观察到的基序。我们观察到在化反应期间对E- hydr几何形状的偶然动力学偏好,因为只有E-异构体可以进行螯合控制的还原。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号