当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On‐Surface Formation of Cumulene by Dehalogenative Homocoupling of Alkenyl gem‐Dibromides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-08-28 , DOI: 10.1002/anie.201706104
Qiang Sun 1 , Bay V. Tran 2 , Liangliang Cai 1 , Honghong Ma 1 , Xin Yu 1 , Chunxue Yuan 1 , Meike Stöhr 2 , Wei Xu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-08-28 , DOI: 10.1002/anie.201706104
Qiang Sun 1 , Bay V. Tran 2 , Liangliang Cai 1 , Honghong Ma 1 , Xin Yu 1 , Chunxue Yuan 1 , Meike Stöhr 2 , Wei Xu 1
Affiliation
|
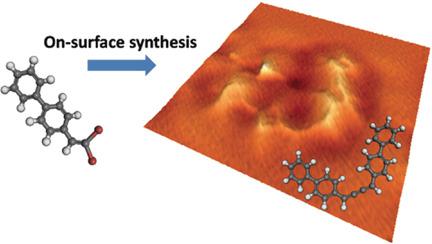
The on‐surface activation of carbon–halogen groups is an efficient route to produce radicals for constructing various hydrocarbons and carbon nanostructures. To date, the employed halide precursors have only one halogen attached to a carbon atom. It is thus of interest to study the effect of attaching more than one halogen atom to a carbon atom with the aim of producing multiple unpaired electrons. By introducing an alkenyl gem‐dibromide, cumulene products were fabricated on a Au(111) surface by dehalogenative homocoupling reactions. The reaction products and pathways were unambiguously characterized by a combination of high‐resolution scanning tunneling microscopy and non‐contact atomic force microscopy measurements together with density functional calculations. This study further supplements the database of on‐surface synthesis strategies and provides a facile manner for incorporation of more complicated carbon scaffolds into surface nanostructures.
中文翻译:

烯基宝石-二溴化物的脱卤均质偶合在Cumulene的表面形成。
碳-卤素基团的表面活化是产生自由基的有效途径,可用于构建各种碳氢化合物和碳纳米结构。迄今为止,所使用的卤化物前体仅具有连接至碳原子的一个卤素。因此,为了产生多个不成对的电子,研究将不止一个卤素原子连接到碳原子上的效果是令人感兴趣的。通过引入烯基宝石二溴化物,通过脱卤均偶联反应在Au(111)表面上制备了异丙苯产品。通过高分辨率扫描隧道显微镜和非接触原子力显微镜测量以及密度泛函计算相结合,明确表征了反应产物和途径。
更新日期:2017-08-28
中文翻译:

烯基宝石-二溴化物的脱卤均质偶合在Cumulene的表面形成。
碳-卤素基团的表面活化是产生自由基的有效途径,可用于构建各种碳氢化合物和碳纳米结构。迄今为止,所使用的卤化物前体仅具有连接至碳原子的一个卤素。因此,为了产生多个不成对的电子,研究将不止一个卤素原子连接到碳原子上的效果是令人感兴趣的。通过引入烯基宝石二溴化物,通过脱卤均偶联反应在Au(111)表面上制备了异丙苯产品。通过高分辨率扫描隧道显微镜和非接触原子力显微镜测量以及密度泛函计算相结合,明确表征了反应产物和途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号