Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-08-04 , DOI: 10.1016/j.bmc.2017.08.002 Kun Li , Tianyi Ma , Jingjing Cai , Min Huang , Hongye Guo , Di Zhou , Shenglin Luan , Jinyu Yang , Dan Liu , Yongkui Jing , Linxiang Zhao
|
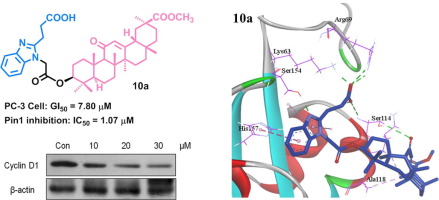
Twenty-six conjugates of 18β-glycyrrhetinic acid derivatives with 3-(1H-benzo[d]imidazol-2-yl)propanoic acid were designed and synthesized as Pin1 inhibitors. Most of these semi-synthetic compounds showed improved Pin1 inhibitory activity and anti-proliferative effects against prostate cancer cells as compared to 3-(1H-benzo[d]imidazol-2-yl)propanoic acid and GA. Compounds 10a and 12i were the most potent to inhibit growth of prostate cancer PC-3 with GI50 values of 7.80 μM and 3.52 μM, respectively. The enzyme inhibition ratio of nine compounds at 10 μM was over 90%. Structure-activity relationships indicated that both appropriate structure at ring C of GA and suitable length of linker between GA skeleton and benzimidazole moiety had significant impact on improving activity. Western blot assay revealed that 10a decreased the level of cell cycle regulating protein cyclin D1. Thus, these compounds might represent a novel anti-proliferative agent working through Pin1 inhibition.
中文翻译:

18种β-甘草次酸衍生物与3-(1 H-苯并[ d ]咪唑-2-基)丙酸作为Pin1抑制剂的结合物,具有抗前列腺癌的能力
二十六个18个缀合物β与3-(1β-甘草酸衍生物ħ -苯并[ d ]咪唑-2-基)丙酸,设计并作为中Pin1抑制剂合成。与3-(1 H-苯并[ d ]咪唑-2-基)丙酸和GA相比,这些半合成化合物大多数显示出改善的Pin1抑制活性和对前列腺癌细胞的抗增殖作用。化合物10a和12i最有效地抑制GI 50抑制前列腺癌PC-3的生长值分别为7.80μM和3.52μM。9种化合物在10μM时的酶抑制率超过90%。构效关系表明,GA环C的合适结构以及GA骨架与苯并咪唑部分之间合适的接头长度对提高活性均具有重要影响。蛋白质印迹分析显示10a降低了细胞周期调节蛋白cyclin D1的水平。因此,这些化合物可能代表了通过抑制Pin1起作用的新型抗增殖剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号