当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Temperature and Surfactants on the Solubilization of Hexachlorobutadiene and Hexachloroethane
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.jced.7b00320
Romain Rodrigues 1, 2, 3 , Stéphanie Betelu 1 , Stéfan Colombano 1 , Guillaume Masselot 2 , Theodore Tzedakis 3 , Ioannis Ignatiadis 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.jced.7b00320
Romain Rodrigues 1, 2, 3 , Stéphanie Betelu 1 , Stéfan Colombano 1 , Guillaume Masselot 2 , Theodore Tzedakis 3 , Ioannis Ignatiadis 1
Affiliation
|
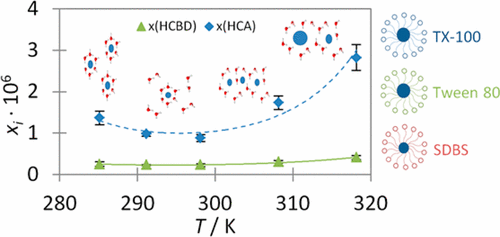
The solubilization of hexachlorobutadiene (HCBD) and hexachloroethane (HCA) in water as a function of temperature and in the presence of surfactants was investigated in order to predict their fate in groundwater and to increase their recovery. HCBD and HCA solubility data were experimentally determined at five temperatures in the range from (285.15 to 318.15) K. Thermodynamic parameters for dissolution (ΔsolG°, ΔsolH°, and ΔsolS°) have been calculated in order to propose a physical explanation of the minimum solubility observed between 293.15 and 298.15 K for both compounds. The solubilization process appeared to be influenced by the network of water molecules rather than by physical and chemical properties of HCBD or HCA, due to an opposite effect of temperature onto Brownian motion, which increases with temperature, and hydrogen-bond network, which collapses with temperature. Concerning the influence of surfactants, determination of the micelle–water partition coefficients (Kmw) and the molar solubilization ratio (MSR) has shown that the solubilization per micelle was more important for nonionic surfactants Triton X-100 and Tween 80 than for anionic SDBS. Also, the increase of solubility was 1 order of magnitude higher for liquid HCBD than for crystalline HCA irrespective of surfactant.
中文翻译:

温度和表面活性剂对六氯丁二烯和六氯乙烷增溶的影响
研究了六氯丁二烯(HCBD)和六氯乙烷(HCA)在水中的溶解度与温度的关系,并在存在表面活性剂的情况下进行了研究,目的是预测它们在地下水中的命运并提高其回收率。六氯丁二烯和HCA溶解度数据在从(285.15到318.15)溶解K.热力学参数(Δ范围5度的温度进行了实验测定溶胶ģ °,Δ溶胶ħ °,Δ溶胶小号计算°)是为了对两种化合物在293.15和298.15 K之间观察到的最小溶解度提出物理解释。溶解过程似乎受水分子网络的影响,而不是受HCBD或HCA的物理和化学性质的影响,这是由于温度对布朗运动的反作用(随温度而增加)和氢键网络(随氢而崩溃)所致。温度。关于表面活性剂的影响,胶束-水分配系数的确定(K mw)和摩尔增溶比(MSR)表明,对于非离子表面活性剂Triton X-100和Tween 80,每胶束的增溶比对阴离子SDBS更重要。同样,与表面活性剂无关,液态HCBD的溶解度增加比结晶态的HCA高1个数量级。
更新日期:2017-08-03
中文翻译:

温度和表面活性剂对六氯丁二烯和六氯乙烷增溶的影响
研究了六氯丁二烯(HCBD)和六氯乙烷(HCA)在水中的溶解度与温度的关系,并在存在表面活性剂的情况下进行了研究,目的是预测它们在地下水中的命运并提高其回收率。六氯丁二烯和HCA溶解度数据在从(285.15到318.15)溶解K.热力学参数(Δ范围5度的温度进行了实验测定溶胶ģ °,Δ溶胶ħ °,Δ溶胶小号计算°)是为了对两种化合物在293.15和298.15 K之间观察到的最小溶解度提出物理解释。溶解过程似乎受水分子网络的影响,而不是受HCBD或HCA的物理和化学性质的影响,这是由于温度对布朗运动的反作用(随温度而增加)和氢键网络(随氢而崩溃)所致。温度。关于表面活性剂的影响,胶束-水分配系数的确定(K mw)和摩尔增溶比(MSR)表明,对于非离子表面活性剂Triton X-100和Tween 80,每胶束的增溶比对阴离子SDBS更重要。同样,与表面活性剂无关,液态HCBD的溶解度增加比结晶态的HCA高1个数量级。

































 京公网安备 11010802027423号
京公网安备 11010802027423号