当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Apoferritin Nanocage for Brain Targeted Doxorubicin Delivery
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00341 Zhijiang Chen 1, 2, 3 , Meifang Zhai 1, 2, 4 , Xiangyang Xie 5 , Yue Zhang 1, 2, 5 , Siyu Ma 1, 2 , Zhiping Li 1, 2 , Fanglin Yu 1, 2 , Baoquan Zhao 1, 2 , Min Zhang 1, 2 , Yang Yang 1, 2 , Xingguo Mei 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-08-02 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00341 Zhijiang Chen 1, 2, 3 , Meifang Zhai 1, 2, 4 , Xiangyang Xie 5 , Yue Zhang 1, 2, 5 , Siyu Ma 1, 2 , Zhiping Li 1, 2 , Fanglin Yu 1, 2 , Baoquan Zhao 1, 2 , Min Zhang 1, 2 , Yang Yang 1, 2 , Xingguo Mei 1, 2
Affiliation
|
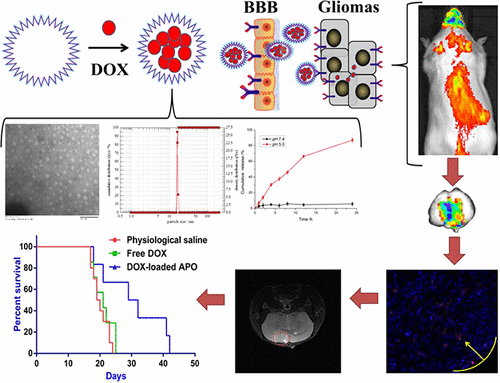
An ideal brain-targeted nanocarrier must be sufficiently potent to penetrate the blood–brain barrier (BBB) and sufficiently competent to target the cells of interest with adequate optimized physiochemical features and biocompatibility. However, it is an enormous challenge to the researchers to organize the above-mentioned properties into a single nanocarrier particle. New frontiers in nanomedicine are advancing the research of new biomaterials. Herein, we demonstrate a straightforward strategy for brain targeting by encapsulating doxorubicin (DOX) into a naturally available and unmodified apoferritin nanocage (DOX-loaded APO). APO can specifically bind to cells expressing transferrin receptor 1 (TfR1). Because of the high expression of TfR1 in both brain endothelial and glioma cells, DOX-loaded APO can cross the BBB and deliver drugs to the glioma with TfR1. Subsequent research demonstrated that the DOX-loaded APO had good physicochemical properties (particle size of 12.03 ± 0.42 nm, drug encapsulation efficiency of 81.8 ± 1.1%) and significant penetrating and targeting effects in the coculture model of bEnd.3 and C6 cells in vitro. In vivo imaging revealed that DOX-loaded APO accumulated specifically in brain tumor tissues. Additionally, in vivo tumor therapy experiments (at a dosage of 1 mg/kg DOX) demonstrated that a longer survival period was observed in mice that had been treated with DOX-loaded APO (30 days) compared with mice receiving free DOX solution (19 days).
中文翻译:

阿朴铁蛋白纳米笼用于脑靶向阿霉素的递送
理想的脑靶向纳米载体必须具有足够的能力穿透血脑屏障(BBB),并具有足够的理化特性和生物相容性,足以靶向目标细胞。然而,将上述性质组织成单个纳米载体颗粒对研究人员是巨大的挑战。纳米医学的新领域正在推进新生物材料的研究。在本文中,我们通过将阿霉素(DOX)封装到天然可用且未修饰的载铁蛋白纳米笼(载有DOX的APO)中,展示了一种针对大脑的直接策略。APO可以与表达转铁蛋白受体1(TfR1)的细胞特异性结合。由于TfR1在脑内皮细胞和神经胶质瘤细胞中高表达,载有DOX的APO可以穿过血脑屏障,并通过TfR1将药物递送至神经胶质瘤。随后的研究表明,载有DOX的APO具有良好的理化特性(粒径为12.03±0.42 nm,药物包封效率为81.8±1.1%),并且在bEnd.3和C6细胞的共培养模型中具有显着的穿透和靶向作用体外。体内成像显示,装载DOX的APO专门在脑肿瘤组织中积累。此外,体内肿瘤治疗实验(剂量为1 mg / kg DOX)表明,与接受游离DOX溶液的小鼠相比,接受过DOX的APO治疗的小鼠(30天)观察到更长的生存期(19)。天)。
更新日期:2017-08-03
中文翻译:

阿朴铁蛋白纳米笼用于脑靶向阿霉素的递送
理想的脑靶向纳米载体必须具有足够的能力穿透血脑屏障(BBB),并具有足够的理化特性和生物相容性,足以靶向目标细胞。然而,将上述性质组织成单个纳米载体颗粒对研究人员是巨大的挑战。纳米医学的新领域正在推进新生物材料的研究。在本文中,我们通过将阿霉素(DOX)封装到天然可用且未修饰的载铁蛋白纳米笼(载有DOX的APO)中,展示了一种针对大脑的直接策略。APO可以与表达转铁蛋白受体1(TfR1)的细胞特异性结合。由于TfR1在脑内皮细胞和神经胶质瘤细胞中高表达,载有DOX的APO可以穿过血脑屏障,并通过TfR1将药物递送至神经胶质瘤。随后的研究表明,载有DOX的APO具有良好的理化特性(粒径为12.03±0.42 nm,药物包封效率为81.8±1.1%),并且在bEnd.3和C6细胞的共培养模型中具有显着的穿透和靶向作用体外。体内成像显示,装载DOX的APO专门在脑肿瘤组织中积累。此外,体内肿瘤治疗实验(剂量为1 mg / kg DOX)表明,与接受游离DOX溶液的小鼠相比,接受过DOX的APO治疗的小鼠(30天)观察到更长的生存期(19)。天)。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号