European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-08-01 , DOI: 10.1016/j.ejmech.2017.07.071 Nobuki Sakauchi , Hideki Furukawa , Junya Shirai , Ayumu Sato , Haruhiko Kuno , Reiko Saikawa , Masato Yoshida
|
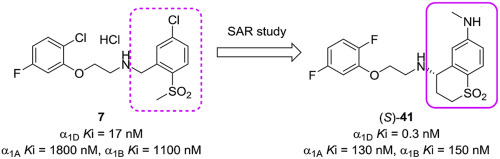
A series of phenoxyethylamine derivatives was designed and synthesized to discover potent and selective human α1D adrenoceptor (α1D adrenergic receptor; α1D–AR) antagonists. Compound 7 was taken from our internal compound collection as an attractive starting point and exhibited moderate binding affinity for α1D–AR and high selectivity against α1A– and α1B–ARs. We focused on modifying the 2-methylsulfonylbenzyl group of 7 to discover novel compounds structurally distinct from other reported α1–AR antagonists containing the phenoxyethylamine motif. Study of structure activity relationship guided by a targeted ligand-lipophilicity efficiency score led to the discovery of a novel scaffold of 3,4-dihydro-2H-thiochromene 1,1-dioxide for selective α1D–AR antagonists. Further optimization studies resulted in the identification of (4S)-N4-[2-(2,5-difluorophenoxy)ethyl]-N6-methyl-3,4-dihydro-2H-thiochromene-4,6-diamine 1,1-dioxide, (S)–41, as a novel, highly potent and selective α1D–AR antagonist. Herein, we provide details of the structure activity relationship of the phenoxyethylamine analog for the potency and selectivity.
中文翻译:

3,4-二氢2的识别ħ与苯氧基乙基胺基团作为高度有效-thiochromene -1,1-二氧化物衍生物和选择性α 1D肾上腺素受体拮抗剂
一系列苯氧基乙基胺衍生物的设计和合成以发现有效的和选择性的人α 1D肾上腺素受体(α 1D肾上腺素能受体;α 1D -AR)拮抗剂。化合物7是从我们的内部化合物集合作为一个有吸引力的起点和表现出中度的结合亲和性为α 1D -AR和针对α高选择性1A -和α 1B -ARs。我们集中在修改的2-甲基磺酰基苄基7来发现从其它报道的α结构上不同的新的化合物1含有苯氧乙胺基序的–AR拮抗剂。通过有针对性的配体-亲油性效率引导结构活性关系研究得分导致3,4-二氢2的新型支架的发现ħ -thiochromene -1,1-二氧化物进行选择性α 1D -AR拮抗剂。进一步的优化研究确定了(4 S)-N 4- [2-(2,5-二氟苯氧基)乙基] -N 6-甲基-3,4-二氢-2 H-硫代色素-4,6-二胺1,1-dioxide((S)– 41)作为一种新型的,高效且有选择性的α1D–AR拮抗剂。在本文中,我们提供了有关苯氧基乙胺类似物的效价和选择性的结构活性关系的详细信息。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号