当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Future prospect of insulin inhalation for diabetic patients: The case of Afrezza versus Exubera
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2015-08-07 09:10:55 Moawia M. Al-Tabakha
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2015-08-07 09:10:55 Moawia M. Al-Tabakha
|
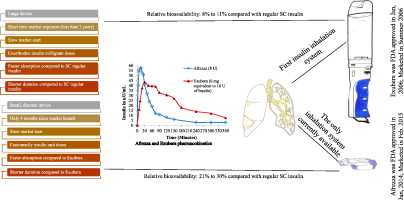
The current review was designed to compare between the insulin inhalation systems Exubera and Afrezza and to investigate the reasons why Exubera was unsuccessful, when Afrezza maker is expecting their product to be felicitous. In January 2006, Pfizer secured FDA and EC approval for the first of its kind, regular insulin through Exubera inhaler device for the management of types 1 and 2 diabetes mellitus (DM) in adults. The product was no longer available to the market after less than two years from its approval triggering a setback for competitive new inhalable insulins that were already in various clinical development phases. In contrary, MannKind Corporation started developing its ultra-rapid-acting insulin Afrezza in a bold bid, probably by managing the issues in which Exubera was not successful. Afrezza has been marketed since February, 2015 by Sanofi after getting FDA approval in June 2014. The results from this systematic review indicate the effectiveness of insulin inhalation products, particularly for patients initiating insulin therapy. Pharmaceutical companies should capitalize on the information available from insulin inhalation to produce competitive products that are able to match the bioavailability of subcutaneous (SC) insulin injection and to deal with the single insulin unit increments and basal insulin requirements in some diabetic patients or extending the horizon to inhalable drug products with completely different drug entities for other indications.
中文翻译:

糖尿病患者吸入胰岛素的未来前景:Afrezza与Exubera的案例
本篇综述旨在比较胰岛素吸入系统Exubera和Afrezza之间的差异,并调查当Afrezza制造商期望其产品合格时Exubera不成功的原因。2006年1月,辉瑞通过Exubera吸入器设备获得了FDA和EC的首款常规胰岛素,用于成人1型和2型糖尿病(DM)的管理。该产品自获得批准之日起不到两年后就不再投放市场,这已经使竞争性可吸入新胰岛素遭受挫折,而这些新可吸入胰岛素已经处于各个临床开发阶段。相反,MannKind公司以大胆的出价开始开发其超速效胰岛素Afrezza,可能是通过解决Exubera未能成功解决的问题。Afrezza自2月份开始投放市场,在2014年6月获得FDA批准后由Sanofi于2015年提出。该系统评价的结果表明胰岛素吸入产品的有效性,特别是对于开始胰岛素治疗的患者。制药公司应利用从胰岛素吸入中获得的信息来生产具有竞争力的产品,这些产品必须能够与皮下(SC)胰岛素注射的生物利用度相匹配,并且能够应对某些糖尿病患者或延长视野的单个胰岛素单位增量和基础胰岛素需求用于其他适应症的具有完全不同药物成分的可吸入药物产品。
更新日期:2015-08-08
中文翻译:

糖尿病患者吸入胰岛素的未来前景:Afrezza与Exubera的案例
本篇综述旨在比较胰岛素吸入系统Exubera和Afrezza之间的差异,并调查当Afrezza制造商期望其产品合格时Exubera不成功的原因。2006年1月,辉瑞通过Exubera吸入器设备获得了FDA和EC的首款常规胰岛素,用于成人1型和2型糖尿病(DM)的管理。该产品自获得批准之日起不到两年后就不再投放市场,这已经使竞争性可吸入新胰岛素遭受挫折,而这些新可吸入胰岛素已经处于各个临床开发阶段。相反,MannKind公司以大胆的出价开始开发其超速效胰岛素Afrezza,可能是通过解决Exubera未能成功解决的问题。Afrezza自2月份开始投放市场,在2014年6月获得FDA批准后由Sanofi于2015年提出。该系统评价的结果表明胰岛素吸入产品的有效性,特别是对于开始胰岛素治疗的患者。制药公司应利用从胰岛素吸入中获得的信息来生产具有竞争力的产品,这些产品必须能够与皮下(SC)胰岛素注射的生物利用度相匹配,并且能够应对某些糖尿病患者或延长视野的单个胰岛素单位增量和基础胰岛素需求用于其他适应症的具有完全不同药物成分的可吸入药物产品。






























 京公网安备 11010802027423号
京公网安备 11010802027423号