当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Electrochemical Reaction of Tin Oxalate-Reduced Graphene Oxide Composite Anode for Rechargeable Lithium Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-07-31 00:00:00 , DOI: 10.1021/acsami.7b03325 Jae-Sang Park 1 , Jae-Hyeon Jo 1 , Hitoshi Yashiro 2 , Sung-Soo Kim 3 , Sun-Jae Kim 1 , Yang-Kook Sun 4 , Seung-Taek Myung 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-07-31 00:00:00 , DOI: 10.1021/acsami.7b03325 Jae-Sang Park 1 , Jae-Hyeon Jo 1 , Hitoshi Yashiro 2 , Sung-Soo Kim 3 , Sun-Jae Kim 1 , Yang-Kook Sun 4 , Seung-Taek Myung 1
Affiliation
|
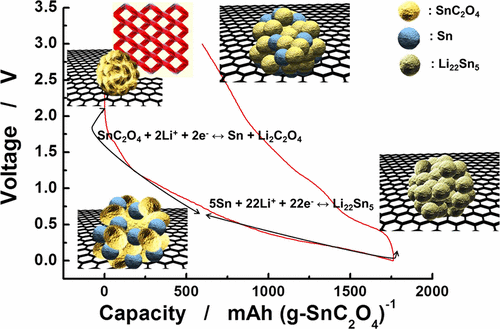
Unlike for SnO2, few studies have reported on the use of SnC2O4 as an anode material for rechargeable lithium batteries. Here, we first introduce a SnC2O4-reduced graphene oxide composite produced via hydrothermal reactions followed by a layer-by-layer self-assembly process. The addition of rGO increased the electric conductivity up to ∼10–3 S cm–1. As a result, the SnC2O4-reduced graphene oxide electrode exhibited a high charge (oxidation) capacity of ∼1166 mAh g–1 at a current of 100 mA g–1 (0.1 C-rate) with a good retention delivering approximately 620 mAh g–1 at the 200th cycle. Even at a rate of 10 C (10 A g–1), the composite electrode was able to obtain a charge capacity of 467 mAh g–1. In contrast, the bare SnC2O4 had inferior electrochemical properties relative to those of the SnC2O4-reduced graphene oxide composite: ∼643 mAh g–1 at the first charge, retaining 192 mAh g–1 at the 200th cycle and 289 mAh g–1 at 10 C. This improvement in electrochemical properties is most likely due to the improvement in electric conductivity, which enables facile electron transfer via simultaneous conversion above 0.75 V and de/alloy reactions below 0.75 V: SnC2O4 + 2Li+ + 2e– → Sn + Li2C2O4 + xLi+ + xe– → LixSn on discharge (reduction) and vice versa on charge. This was confirmed by systematic studies of ex situ X-ray diffraction, transmission electron microscopy, and time-of-flight secondary-ion mass spectroscopy.
中文翻译:

草酸锡还原可充电锂电池氧化石墨烯复合阳极的合成及电化学反应
与SnO 2不同,很少有研究报道将SnC 2 O 4用作可再充电锂电池的负极材料。在这里,我们首先介绍通过水热反应,然后进行逐层自组装工艺生产的SnC 2 O 4还原氧化石墨烯复合材料。添加rGO可将电导率提高到约10 –3 S cm –1。结果,在100 mA g –1的电流下,SnC 2 O 4还原的氧化石墨烯电极显示出约1166 mAh g –1的高电荷(氧化)容量。(0.1 C速率)具有良好的保留能力,在第200个周期时可提供大约620 mAh g –1。即使在10 C(10 A g –1)的速率下,复合电极也能够获得467 mAh g –1的充电容量。相比之下,相对于SnC 2 O 4还原的氧化石墨烯复合材料,SnC 2 O 4裸露的电化学性能较差:第一次充电时约为643 mAh g –1,在第200次循环时保持192 mAh g –1。 289 mAh g –1电化学性能的改善很可能归因于电导率的改善,这可以通过同时转换高于0.75 V和低于0.75 V的脱/合金反应实现便捷的电子转移:SnC 2 O 4 + 2Li + + 2e – →锡+栗2 c ^ 2 Ò 4 + X栗+ + X ë - →锂X的Sn上放电(还原)和反之亦然的电荷。通过异位X射线衍射,透射电子显微镜和飞行时间二次离子质谱的系统研究证实了这一点。
更新日期:2017-07-31
中文翻译:

草酸锡还原可充电锂电池氧化石墨烯复合阳极的合成及电化学反应
与SnO 2不同,很少有研究报道将SnC 2 O 4用作可再充电锂电池的负极材料。在这里,我们首先介绍通过水热反应,然后进行逐层自组装工艺生产的SnC 2 O 4还原氧化石墨烯复合材料。添加rGO可将电导率提高到约10 –3 S cm –1。结果,在100 mA g –1的电流下,SnC 2 O 4还原的氧化石墨烯电极显示出约1166 mAh g –1的高电荷(氧化)容量。(0.1 C速率)具有良好的保留能力,在第200个周期时可提供大约620 mAh g –1。即使在10 C(10 A g –1)的速率下,复合电极也能够获得467 mAh g –1的充电容量。相比之下,相对于SnC 2 O 4还原的氧化石墨烯复合材料,SnC 2 O 4裸露的电化学性能较差:第一次充电时约为643 mAh g –1,在第200次循环时保持192 mAh g –1。 289 mAh g –1电化学性能的改善很可能归因于电导率的改善,这可以通过同时转换高于0.75 V和低于0.75 V的脱/合金反应实现便捷的电子转移:SnC 2 O 4 + 2Li + + 2e – →锡+栗2 c ^ 2 Ò 4 + X栗+ + X ë - →锂X的Sn上放电(还原)和反之亦然的电荷。通过异位X射线衍射,透射电子显微镜和飞行时间二次离子质谱的系统研究证实了这一点。







































 京公网安备 11010802027423号
京公网安备 11010802027423号