当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Initial Reduction of CO2 on Pd-, Ru-, and Cu-Doped CeO2(111) Surfaces: Effects of Surface Modification on Catalytic Activity and Selectivity
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-07-31 00:00:00 , DOI: 10.1021/acsami.7b07945 Chen Guo 1 , Shuxian Wei 1 , Sainan Zhou 1 , Tian Zhang 1 , Zhaojie Wang 1 , Siu-Pang Ng 2 , Xiaoqing Lu 1 , Chi-Man Lawrence Wu 2 , Wenyue Guo 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-07-31 00:00:00 , DOI: 10.1021/acsami.7b07945 Chen Guo 1 , Shuxian Wei 1 , Sainan Zhou 1 , Tian Zhang 1 , Zhaojie Wang 1 , Siu-Pang Ng 2 , Xiaoqing Lu 1 , Chi-Man Lawrence Wu 2 , Wenyue Guo 1
Affiliation
|
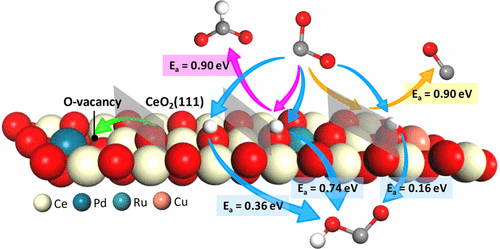
Surface modification by metal doping is an effective treatment technique for improving surface properties for CO2 reduction. Herein, the effects of doped Pd, Ru, and Cu on the adsorption, activation, and reduction selectivity of CO2 on CeO2(111) were investigated by periodic density functional theory. The doped metals distorted the configuration of a perfect CeO2(111) by weakening the adjacent Ce–O bond strength, and Pd doping was beneficial for generating a highly active O vacancy. The analyses of adsorption energy, charge density difference, and density of states confirmed that the doped metals were conducive for enhancing CO2 adsorption, especially for Cu/CeO2(111). The initial reductive dissociation CO2 → CO* + O* on metal-doped CeO2(111) followed the sequence of Cu- > perfect > Pd- > Ru-doped CeO2(111); the reductive hydrogenation CO2 + H → COOH* followed the sequence of Cu- > perfect > Ru- > Pd-doped CeO2(111), in which the most competitive route on Cu/CeO2(111) was exothermic by 0.52 eV with an energy barrier of 0.16 eV; the reductive hydrogenation CO2 + H → HCOO* followed the sequence of Ru- > perfect > Pd-doped CeO2(111). Energy barrier decomposition analyses were performed to identify the governing factors of bond activation and scission along the initial CO2 reduction routes. Results of this study provided deep insights into the effect of surface modification on the initial reduction mechanisms of CO2 on metal-doped CeO2(111) surfaces.
中文翻译:

在Pd,Ru和Cu掺杂的CeO 2(111)表面上最初还原CO 2:表面改性对催化活性和选择性的影响
通过金属掺杂进行的表面改性是用于改善表面性质以减少CO 2的有效处理技术。在本文中,通过周期性密度泛函理论研究了掺杂的Pd,Ru和Cu对CO 2在CeO 2(111)上的吸附,活化和还原选择性的影响。掺杂的金属会通过削弱相邻的Ce-O键强度而扭曲完美的CeO 2(111)的构型,而Pd掺杂有利于产生高活性的O空位。对吸附能,电荷密度差和态密度的分析证实,掺杂金属有利于增强CO 2的吸附,特别是对Cu / CeO 2的吸附。(111)。在金属掺杂的CeO 2(111)上的初始还原解离CO 2 →CO * + O *依次为Cu->完全> Pd-> Ru掺杂的CeO 2(111);还原氢化CO 2 + H→COOH *遵循Cu->完全> Ru-> Pd掺杂的CeO 2(111)的顺序,其中Cu / CeO 2(111)上最具竞争性的途径放热0.52 eV能量垒为0.16 eV; 还原氢化CO 2 + H→HCOO *遵循Ru-> Perfect> Pd掺杂CeO 2(111)的顺序。进行了能垒分解分析,以确定沿初始CO 2的键活化和断裂的控制因素减少路线。这项研究的结果为表面改性对金属掺杂的CeO 2(111)表面上CO 2的初始还原机理的影响提供了深刻的见解。
更新日期:2017-07-31
中文翻译:

在Pd,Ru和Cu掺杂的CeO 2(111)表面上最初还原CO 2:表面改性对催化活性和选择性的影响
通过金属掺杂进行的表面改性是用于改善表面性质以减少CO 2的有效处理技术。在本文中,通过周期性密度泛函理论研究了掺杂的Pd,Ru和Cu对CO 2在CeO 2(111)上的吸附,活化和还原选择性的影响。掺杂的金属会通过削弱相邻的Ce-O键强度而扭曲完美的CeO 2(111)的构型,而Pd掺杂有利于产生高活性的O空位。对吸附能,电荷密度差和态密度的分析证实,掺杂金属有利于增强CO 2的吸附,特别是对Cu / CeO 2的吸附。(111)。在金属掺杂的CeO 2(111)上的初始还原解离CO 2 →CO * + O *依次为Cu->完全> Pd-> Ru掺杂的CeO 2(111);还原氢化CO 2 + H→COOH *遵循Cu->完全> Ru-> Pd掺杂的CeO 2(111)的顺序,其中Cu / CeO 2(111)上最具竞争性的途径放热0.52 eV能量垒为0.16 eV; 还原氢化CO 2 + H→HCOO *遵循Ru-> Perfect> Pd掺杂CeO 2(111)的顺序。进行了能垒分解分析,以确定沿初始CO 2的键活化和断裂的控制因素减少路线。这项研究的结果为表面改性对金属掺杂的CeO 2(111)表面上CO 2的初始还原机理的影响提供了深刻的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号