当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibiotic That Inhibits the ATPase Activity of an ATP-Binding Cassette Transporter by Binding to a Remote Extracellular Site
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1021/jacs.7b04726 Leigh M. Matano 1 , Heidi G. Morris 1 , Anthony R. Hesser 1 , Sara E. S. Martin 1 , Wonsik Lee 1 , Tristan W. Owens 2 , Emaline Laney 1 , Hidemasa Nakaminami 3 , David Hooper 3 , Timothy C. Meredith 4 , Suzanne Walker 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-07-28 00:00:00 , DOI: 10.1021/jacs.7b04726 Leigh M. Matano 1 , Heidi G. Morris 1 , Anthony R. Hesser 1 , Sara E. S. Martin 1 , Wonsik Lee 1 , Tristan W. Owens 2 , Emaline Laney 1 , Hidemasa Nakaminami 3 , David Hooper 3 , Timothy C. Meredith 4 , Suzanne Walker 1
Affiliation
|
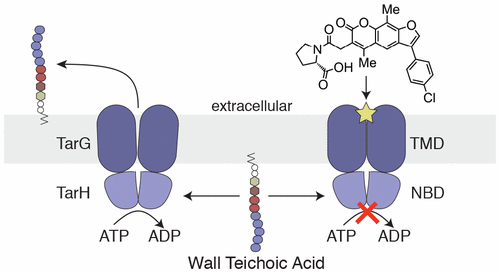
Antibiotic-resistant strains of Staphylococcus aureus pose a major threat to human health and there is an ongoing need for new antibiotics to treat resistant infections. In a high throughput screen (HTS) of 230 000 small molecules designed to identify bioactive wall teichoic acid (WTA) inhibitors, we identified one hit, which was expanded through chemical synthesis into a small panel of potent compounds. We showed that these compounds target TarG, the transmembrane component of the two-component ATP-binding cassette (ABC) transporter TarGH, which exports WTA precursors to the cell surface for attachment to peptidoglycan. We purified, for the first time, a WTA transporter and have reconstituted ATPase activity in proteoliposomes. We showed that this new compound series inhibits TarH-catalyzed ATP hydrolysis even though the binding site maps to TarG near the opposite side of the membrane. These are the first ABC transporter inhibitors shown to block ATPase activity by binding to the transmembrane domain. The compounds have potential as therapeutic agents to treat S. aureus infections, and purification of the transmembrane transporter will enable further development.
中文翻译:

通过结合到远端的细胞外位点,抑制ATP结合盒式转运蛋白的ATPase活性的抗生素。
金黄色葡萄球菌的耐药菌株对人类健康构成重大威胁,并且持续需要新的抗生素来治疗耐药性感染。在旨在识别生物活性壁板壁酸(WTA)抑制剂的23万个小分子的高通量筛选(HTS)中,我们确定了一个命中点,通过化学合成将其扩展为一小撮有效化合物。我们表明,这些化合物靶向TarG,TarG是两组分ATP结合盒(ABC)转运蛋白TarGH的跨膜组分,该转运蛋白将WTA前体输出到细胞表面以附着到肽聚糖上。我们首次纯化了WTA转运蛋白,并在蛋白脂质体中重建了ATPase活性。我们表明,即使结合位点映射到膜相反侧附近的TarG,该新化合物系列也能抑制TarH催化的ATP水解。这些是第一个通过结合跨膜结构域来阻断ATPase活性的ABC转运蛋白抑制剂。该化合物具有作为治疗药物的潜力金黄色葡萄球菌感染和跨膜转运蛋白的纯化将使进一步的发展成为可能。
更新日期:2017-07-30
中文翻译:

通过结合到远端的细胞外位点,抑制ATP结合盒式转运蛋白的ATPase活性的抗生素。
金黄色葡萄球菌的耐药菌株对人类健康构成重大威胁,并且持续需要新的抗生素来治疗耐药性感染。在旨在识别生物活性壁板壁酸(WTA)抑制剂的23万个小分子的高通量筛选(HTS)中,我们确定了一个命中点,通过化学合成将其扩展为一小撮有效化合物。我们表明,这些化合物靶向TarG,TarG是两组分ATP结合盒(ABC)转运蛋白TarGH的跨膜组分,该转运蛋白将WTA前体输出到细胞表面以附着到肽聚糖上。我们首次纯化了WTA转运蛋白,并在蛋白脂质体中重建了ATPase活性。我们表明,即使结合位点映射到膜相反侧附近的TarG,该新化合物系列也能抑制TarH催化的ATP水解。这些是第一个通过结合跨膜结构域来阻断ATPase活性的ABC转运蛋白抑制剂。该化合物具有作为治疗药物的潜力金黄色葡萄球菌感染和跨膜转运蛋白的纯化将使进一步的发展成为可能。































 京公网安备 11010802027423号
京公网安备 11010802027423号