Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-07-29 , DOI: 10.1016/j.bmcl.2017.07.076 Qiu Li , Hai-Kui Yang , Qi Sun , Wen-Wei You , Pei-Liang Zhao
|
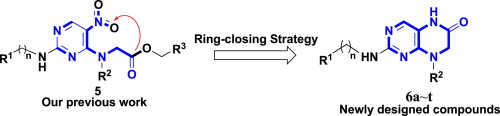
Based on our previous work, a series of novel 2-amino-7,8-dihydropteridin-6(5H)-one derivatives were designed and synthesized via a ring-closing strategy. Biological evaluation with four human cancer cell lines (BT549, T47D, MDA-MB-468, and MDA-MB-231) showed that most of these compounds possessed moderate to potent antiproliferative activities. The most promising compound 8-benzyl-2-(phenethylamino)-7,8-dihydropteridin-6(5H)-one (6q) possessing IC50 values of 7.75, 6.37, and 10.73 μM against MDA-MB-468, T47D, and BT549, respectively, which were 49, 11, and 8 folds more active than the positive control fluorouracil. Moreover, fluorescence-activated cell sorting analysis revealed that compound 6q displayed a significant effect on G1 cell-cycle arrest in a concentration-dependent manner in T47D cells. The initial structure–activity relationship studies indicated that linker-length of amine chain in C-2 position of pyrimidine ring played a crucial role in modulating the antitumor activity, which could be of help in the rational design of dihydropteridin-6(5H)-ones as novel anticancer drugs.
中文翻译:

新型取代的2-氨基-7,8-二氢蝶呤-6(5 H)-one衍生物的设计,合成和抗增殖活性
基于我们以前的工作,设计了一系列新颖的2-氨基-7,8-二氢蝶呤-6(5 H)-one衍生物,并通过闭环策略进行了合成。用四种人类癌细胞系(BT549,T47D,MDA-MB-468和MDA-MB-231)进行生物学评估,结果表明,这些化合物大多数都具有中度到强效的抗增殖活性。最有前途的化合物8-苄基-2-(苯乙氨基)-7,8-二氢蝶呤-6(5 H)-一(6q)对MDA-MB-468,T47D的IC 50值为7.75、6.37和10.73μM和BT549的活性分别比阳性对照氟尿嘧啶高49倍,11倍和8倍。此外,荧光激活细胞分选分析表明化合物6q在T47D细胞中以浓度依赖的方式显示了对G1细胞周期停滞的显着影响。初步的结构与活性关系研究表明,嘧啶环C-2位的胺链接头长度在调节抗肿瘤活性中起关键作用,这可能有助于二氢蝶呤-6(5 H)的合理设计。-一种新的抗癌药。































 京公网安备 11010802027423号
京公网安备 11010802027423号