当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Excited-State Intramolecular Proton Transfer for 1,2-Dihydroxyanthraquinone: Effect of Water on the ESIPT
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/acs.jpca.7b03877 Yajing Peng 1, 2 , Yuqing Ye 1 , Xianming Xiu 1 , Shuang Sun 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/acs.jpca.7b03877 Yajing Peng 1, 2 , Yuqing Ye 1 , Xianming Xiu 1 , Shuang Sun 1
Affiliation
|
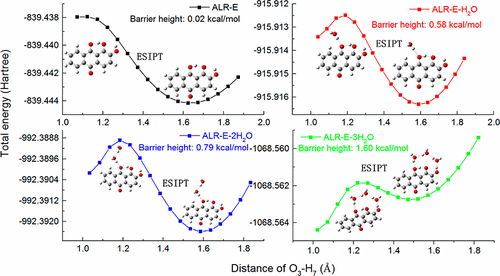
Mechanisms of excited-state intramolecular proton transfer (ESIPT) of 1,2-dihydroxyanthraquinone (ALR) in ethanol solvent and binary solvent of water and ethanol are investigated using the density functional theory and time-dependent density functional theory. The intramolecular hydrogen bond is found to be reinforced in the excited state based on the bond lengths, bond angles, and infrared vibrational spectra of relevant group. The reinforcement of intramolecular hydrogen bond is attributed to the charge transfer in the excited state, which leads the ESIPT to form a keto isomer. The absorption and fluorescence spectra of ALR in binary solvent with different water percentage are obtained and demonstrate the inhibition effect of water on the ESIPT process, which are consistent with the experimentally observation. Furthermore, more water molecules are considered near the carbonyl group and hydroxyl group related to the intramolecular proton transfer to form intermolecular hydrated hydrogen bond with ALR for clarifying the block mechanism of water on ESIPT. The potential energy curves, frontier molecular orbitals, and NBO analysis are calculated for the several complexes in the ground and excited states. The results show that the interrupt role of water on the ESIPT originated from the forming of hydrated hydrogen bond between the carbonyl oxygen atom and the water molecule, which weakens the intramolecular hydrogen bond associated with proton transfer, increases the energy barrier of ESIPT, and thus precludes the transition of ALR-E to ALR-K in the excited state. In addition, the weakening of intramolecular hydrogen bonds is increased as the water molecule number increases. So the inhibitory effect is enhanced by the water quantity, which reasonably explains the experimental attenuating of keto emission spectra as the water percentage in binary solvent increases.
中文翻译:

1,2-二羟基蒽醌的激发态分子内质子转移机理:水对ESIPT的影响
利用密度泛函理论和时变密度泛函理论研究了1,2-二羟基蒽醌(ALR)在乙醇溶剂以及水和乙醇的二元溶剂中的激发态分子内质子转移(ESIPT)的机理。根据相关基团的键长,键角和红外振动光谱,发现分子内氢键在激发态下得到增强。分子内氢键的增强归因于处于激发态的电荷转移,这导致ESIPT形成酮异构体。获得了不同水含量的二元溶剂中ALR的吸收光谱和荧光光谱,证明了水对ESIPT过程的抑制作用,与实验结果相吻合。此外,更多的水分子被认为与分子内质子转移相关的羰基和羟基附近,与ALR形成分子间水合氢键,以阐明水在ESIPT上的阻断机理。计算了基态和激发态下的几种配合物的势能曲线,前沿分子轨道和NBO分析。结果表明,水对ESIPT的阻断作用源于羰基氧原子与水分子之间水合氢键的形成,削弱了与质子转移相关的分子内氢键,增加了ESIPT的能垒,因此排除了在激发态下ALR-E向ALR-K的转变。此外,随着水分子数目的增加,分子内氢键的弱化作用增加。因此,水的量会增强抑制作用,这可以合理地解释当二元溶剂中水的含量增加时,酮发射光谱的实验衰减。
更新日期:2017-07-28
中文翻译:

1,2-二羟基蒽醌的激发态分子内质子转移机理:水对ESIPT的影响
利用密度泛函理论和时变密度泛函理论研究了1,2-二羟基蒽醌(ALR)在乙醇溶剂以及水和乙醇的二元溶剂中的激发态分子内质子转移(ESIPT)的机理。根据相关基团的键长,键角和红外振动光谱,发现分子内氢键在激发态下得到增强。分子内氢键的增强归因于处于激发态的电荷转移,这导致ESIPT形成酮异构体。获得了不同水含量的二元溶剂中ALR的吸收光谱和荧光光谱,证明了水对ESIPT过程的抑制作用,与实验结果相吻合。此外,更多的水分子被认为与分子内质子转移相关的羰基和羟基附近,与ALR形成分子间水合氢键,以阐明水在ESIPT上的阻断机理。计算了基态和激发态下的几种配合物的势能曲线,前沿分子轨道和NBO分析。结果表明,水对ESIPT的阻断作用源于羰基氧原子与水分子之间水合氢键的形成,削弱了与质子转移相关的分子内氢键,增加了ESIPT的能垒,因此排除了在激发态下ALR-E向ALR-K的转变。此外,随着水分子数目的增加,分子内氢键的弱化作用增加。因此,水的量会增强抑制作用,这可以合理地解释当二元溶剂中水的含量增加时,酮发射光谱的实验衰减。































 京公网安备 11010802027423号
京公网安备 11010802027423号