当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism and Rate of Thermal Decomposition of Hexachlorocyclopentadiene and Its Importance in PCDD/F Formation from the Combustion of Cyclodiene Pesticides
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/acs.jpca.7b05209 Nirmala K. Dharmarathne 1 , John C. Mackie 1 , Eric M. Kennedy 1 , Michael Stockenhuber 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-07-26 00:00:00 , DOI: 10.1021/acs.jpca.7b05209 Nirmala K. Dharmarathne 1 , John C. Mackie 1 , Eric M. Kennedy 1 , Michael Stockenhuber 1
Affiliation
|
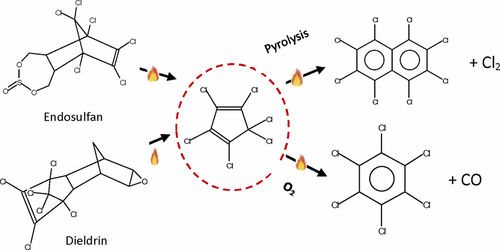
Thermal decomposition of hexachlorocyclopentadiene (HCCP) has been studied in inert gas and under oxidative conditions in a silica flow reactor at a residence time of 5.0 s between 690 and 923 K and 1 atm pressure. Pyrolysis was initiated by Cl bond fission to form pentachlorocyclopentadienyl radical; two such radicals then combined to undergo a series of intramolecular rearrangements and Cl fissions, producing principally octachloronaphthalene (8ClNP) and Cl2. This process has been studied by quantum chemical calculation, and a reaction potential energy surface has been developed. The rate constant of initial Cl atom fission has been calculated by canonical variational transition state theory as k = 1.45 × 1015 exp(−222 ± 9 kJ mol–1/RT) s–1 between 500 and 2000 K. A minimal kinetic model was developed to model the decomposition and major products. Oxidative decomposition was studied in nitrogen with O2 contents of 1, 6, 12, and 20 mol %. Increasing O2 to 6–8% increased the rate of decomposition of HCCP and decreased the yield of 8ClNP. Above 823 K, hexachlorobenzene (HCB) and CO became major products. The oxidative reaction has also been studied quantum chemically. At high O2 content (>∼10%), the rate of decomposition of HCCP declined as did yields of 8ClNP and HCB, but CO yields increased.
中文翻译:

六氯环戊二烯热分解的机理,速率及其在环二烯类农药燃烧中PCDD / F形成中的重要性
已经研究了六氯环戊二烯(HCCP)在惰性气体中和氧化条件下在硅胶流动反应器中在690至923 K和1个大气压之间的停留时间为5.0 s时的热分解。通过Cl键裂变引发热解,形成五氯环戊二烯基;然后,两个这样的自由基结合在一起,经历一系列分子内重排和Cl裂变,主要生成八氯萘(8ClNP)和Cl 2。已经通过量子化学计算研究了该过程,并且已经开发了反应势能表面。初始Cl原子裂变的速率常数已通过规范变分过渡状态理论计算为k = 1.45×10 15 exp(-222±9 kJ mol –1/ RT)s –1在500到2000 K之间。建立了最小动力学模型来模拟分解和主要产物。研究了在O 2含量为1、6、12和20 mol%的氮气中的氧化分解。将O 2增加到6–8%会增加HCCP的分解速率,并降低8ClNP的产率。高于823 K时,六氯苯(HCB)和一氧化碳成为主要产品。氧化反应也已经进行了量子化学研究。在高O 2含量(>〜10%)下,HCCP的分解速率下降,而8ClNP和HCB的产率下降,但CO的产率增加。
更新日期:2017-07-28
中文翻译:

六氯环戊二烯热分解的机理,速率及其在环二烯类农药燃烧中PCDD / F形成中的重要性
已经研究了六氯环戊二烯(HCCP)在惰性气体中和氧化条件下在硅胶流动反应器中在690至923 K和1个大气压之间的停留时间为5.0 s时的热分解。通过Cl键裂变引发热解,形成五氯环戊二烯基;然后,两个这样的自由基结合在一起,经历一系列分子内重排和Cl裂变,主要生成八氯萘(8ClNP)和Cl 2。已经通过量子化学计算研究了该过程,并且已经开发了反应势能表面。初始Cl原子裂变的速率常数已通过规范变分过渡状态理论计算为k = 1.45×10 15 exp(-222±9 kJ mol –1/ RT)s –1在500到2000 K之间。建立了最小动力学模型来模拟分解和主要产物。研究了在O 2含量为1、6、12和20 mol%的氮气中的氧化分解。将O 2增加到6–8%会增加HCCP的分解速率,并降低8ClNP的产率。高于823 K时,六氯苯(HCB)和一氧化碳成为主要产品。氧化反应也已经进行了量子化学研究。在高O 2含量(>〜10%)下,HCCP的分解速率下降,而8ClNP和HCB的产率下降,但CO的产率增加。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号