当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of the TyrOH•+ Radical Cation in the Flavoenzyme TrmFO
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-08 , DOI: 10.1021/jacs.7b04586 Lipsa Nag 1 , Pierre Sournia 1 , Hannu Myllykallio 1 , Ursula Liebl 1 , Marten H. Vos 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-08-08 , DOI: 10.1021/jacs.7b04586 Lipsa Nag 1 , Pierre Sournia 1 , Hannu Myllykallio 1 , Ursula Liebl 1 , Marten H. Vos 1
Affiliation
|
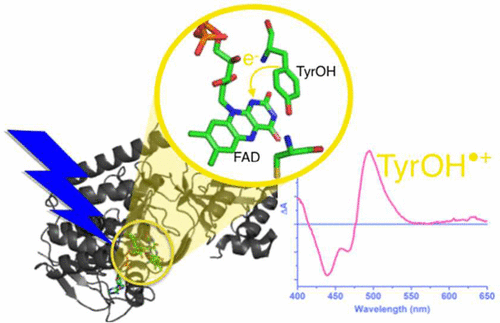
Tyrosine (TyrOH) and tryptophan radicals play important roles as intermediates in biochemical charge-transfer reactions. Tryptophanyl radicals have been observed both in their protonated cation form and in their unprotonated neutral form, but to date, tyrosyl radicals have only been observed in their unprotonated form. With a genetically modified form of the flavoenzyme TrmFO as a suitable model system and using ultrafast fluorescence and absorption spectroscopy, we characterize its protonated precursor TyrOH•+, and we show this species to have a distinct visible absorption band and a transition moment that we suggest to lie close to the phenol symmetry axis. TyrOH•+ is formed in ∼1 ps by electron transfer to excited flavin and decays in ∼3 ps by charge recombination. These findings imply that TyrOH oxidation does not necessarily induce its concerted deprotonation. Our results will allow disentangling of photoproduct states in flavoproteins in often-encountered complex situations and more generally are important for understanding redox chains relying on tyrosyl intermediates.
中文翻译:

黄素酶 TrmFO 中 TyrOH•+ 自由基阳离子的鉴定
酪氨酸 (TyrOH) 和色氨酸自由基在生化电荷转移反应中作为中间体起着重要作用。在质子化的阳离子形式和未质子化的中性形式中都观察到色氨酸自由基,但迄今为止,仅在未质子化的形式中观察到酪氨酰基自由基。使用转基因形式的黄素酶 TrmFO 作为合适的模型系统,并使用超快荧光和吸收光谱,我们表征了其质子化前体 TyrOH•+,并表明该物种具有明显的可见吸收带和我们建议的过渡时刻靠近苯酚对称轴。TyrOH•+ 通过电子转移到激发的黄素在约 1 ps 内形成,并在约 3 ps 内通过电荷复合衰变。这些发现意味着 TyrOH 氧化不一定会诱导其协同去质子化。我们的结果将允许在经常遇到的复杂情况下解开黄素蛋白中的光产物状态,并且更普遍地对于理解依赖于酪氨酰中间体的氧化还原链很重要。
更新日期:2017-08-08
中文翻译:

黄素酶 TrmFO 中 TyrOH•+ 自由基阳离子的鉴定
酪氨酸 (TyrOH) 和色氨酸自由基在生化电荷转移反应中作为中间体起着重要作用。在质子化的阳离子形式和未质子化的中性形式中都观察到色氨酸自由基,但迄今为止,仅在未质子化的形式中观察到酪氨酰基自由基。使用转基因形式的黄素酶 TrmFO 作为合适的模型系统,并使用超快荧光和吸收光谱,我们表征了其质子化前体 TyrOH•+,并表明该物种具有明显的可见吸收带和我们建议的过渡时刻靠近苯酚对称轴。TyrOH•+ 通过电子转移到激发的黄素在约 1 ps 内形成,并在约 3 ps 内通过电荷复合衰变。这些发现意味着 TyrOH 氧化不一定会诱导其协同去质子化。我们的结果将允许在经常遇到的复杂情况下解开黄素蛋白中的光产物状态,并且更普遍地对于理解依赖于酪氨酰中间体的氧化还原链很重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号