当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversibility of Ketone Reduction by SmI2–Water and Formation of Organosamarium Intermediates
Organometallics ( IF 2.5 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/acs.organomet.7b00392
Tesia V. Chciuk 1 , William R. Anderson 1 , Robert A. Flowers 1
Organometallics ( IF 2.5 ) Pub Date : 2017-07-25 00:00:00 , DOI: 10.1021/acs.organomet.7b00392
Tesia V. Chciuk 1 , William R. Anderson 1 , Robert A. Flowers 1
Affiliation
|
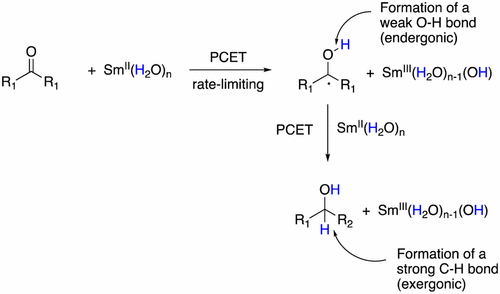
The reduction of ketones by SmI2–water has long been thought to proceed through a reversible initial electron transfer with the formation of organosamarium intermediates in a follow-up step. Kinetic experiments on the reduction of two model ketones and structurally similar ketones with a pendant alkene are shown to be consistent with a rate-limiting reduction by SmI2–water through a proton-coupled electron-transfer (PCET). Literature values for the rates of radical cyclizations and reduction of radicals by SmI2 and thermochemical data for radical reduction by SmI2–water further support a rate-limiting initial step for ketone reductions. These data suggest that discrete organosamarium species may not be intermediates in ketone reductions by SmI2–water.
中文翻译:

SmI 2-水还原酮的可逆性和有机osa中间体的形成
长期以来,人们一直认为通过SmI 2-水还原酮是通过可逆的初始电子转移进行的,并在后续步骤中形成了有机sa中间体。动力学实验表明,使用侧链烯烃还原两个模型酮和结构相似的酮,与通过质子偶联电子转移(PCET)的SmI 2-水限速还原反应是一致的。用于自由基环化的速率和通过SMI减少自由基的文献值2和用于自由基还原热化学数据通过SMI 2-水进一步支持减少酮的限速起始步骤。这些数据表明,离散的有机sa可能不是SmI 2-水还原酮的中间体。
更新日期:2017-07-26
中文翻译:

SmI 2-水还原酮的可逆性和有机osa中间体的形成
长期以来,人们一直认为通过SmI 2-水还原酮是通过可逆的初始电子转移进行的,并在后续步骤中形成了有机sa中间体。动力学实验表明,使用侧链烯烃还原两个模型酮和结构相似的酮,与通过质子偶联电子转移(PCET)的SmI 2-水限速还原反应是一致的。用于自由基环化的速率和通过SMI减少自由基的文献值2和用于自由基还原热化学数据通过SMI 2-水进一步支持减少酮的限速起始步骤。这些数据表明,离散的有机sa可能不是SmI 2-水还原酮的中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号